Deposition Date
2024-06-06
Release Date
2024-11-13
Last Version Date
2025-01-22
Entry Detail
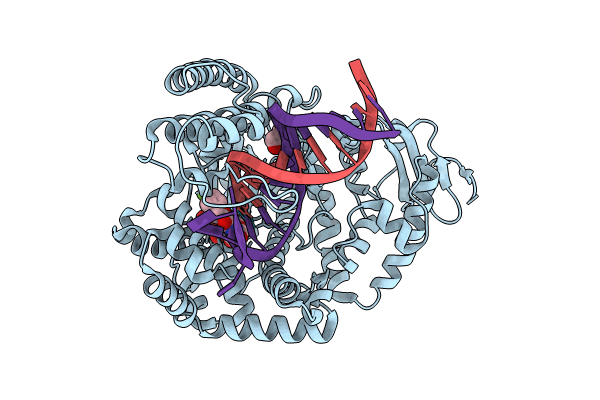
PDB ID:
9FMF
Keywords:
Title:
KlenTaq DNA polymerase in a ternary complex with primer/template and a fluorobenzofuran-modified dUTP (FBFdUTP)
Biological Source:
Source Organism(s):
Thermus aquaticus (Taxon ID: 271)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.10 Å
R-Value Free:
0.23
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 31 2 1


