Deposition Date
2024-03-12
Release Date
2025-03-26
Last Version Date
2025-05-21
Entry Detail
PDB ID:
9EMZ
Keywords:
Title:
13C/15N-labelled Integral Membrane Enzyme LspA in the Lipid Cubic Phase
Biological Source:
Source Organism(s):
Pseudomonas aeruginosa PAO1 (Taxon ID: 208964)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
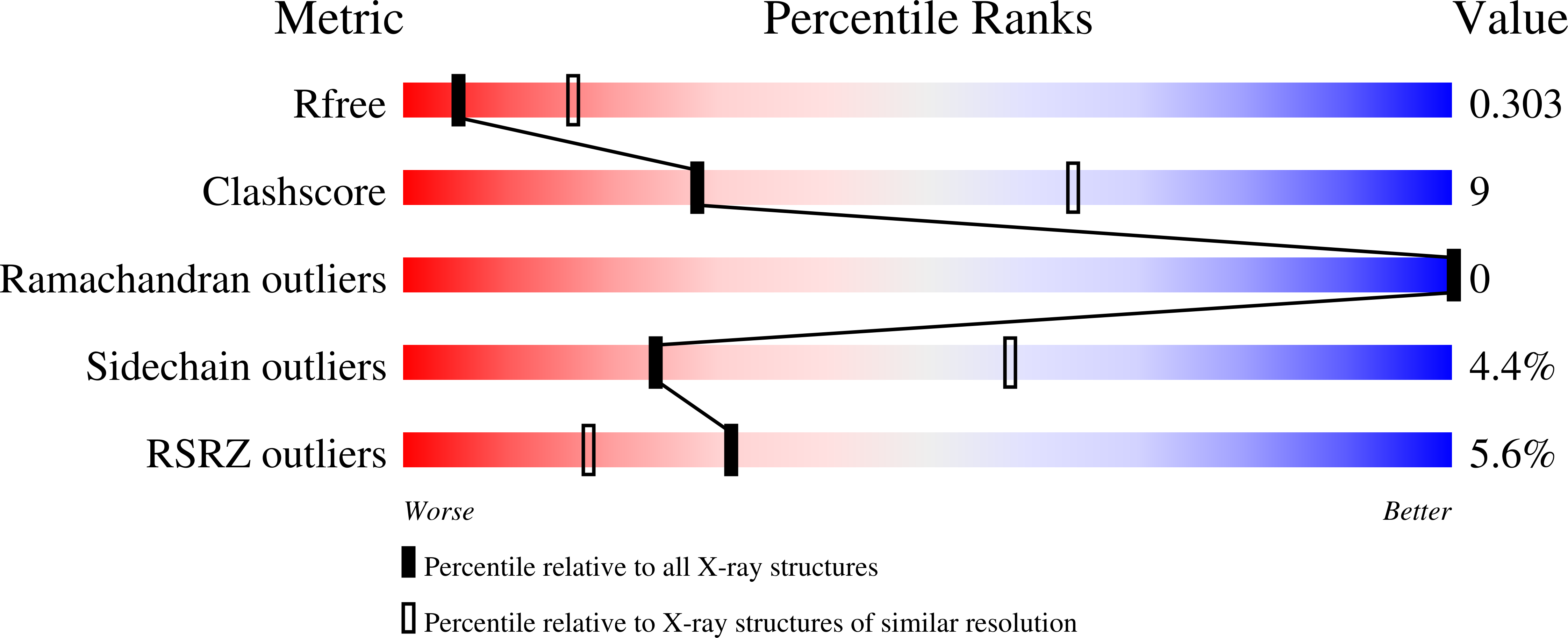
Resolution:
3.00 Å
R-Value Free:
0.30
R-Value Work:
0.24
R-Value Observed:
0.24
Space Group:
C 1 2 1