Deposition Date
2024-11-13
Release Date
2025-03-19
Last Version Date
2025-03-19
Entry Detail
PDB ID:
9EBX
Keywords:
Title:
Chimeric fluorescence biosensor formed from a lactate-binding protein and GFP
Biological Source:
Source Organism(s):
Aequorea victoria (Taxon ID: 6100)
Helicobacter pylori (Taxon ID: 85962)
Helicobacter pylori (Taxon ID: 85962)
Expression System(s):
Method Details:
Experimental Method:
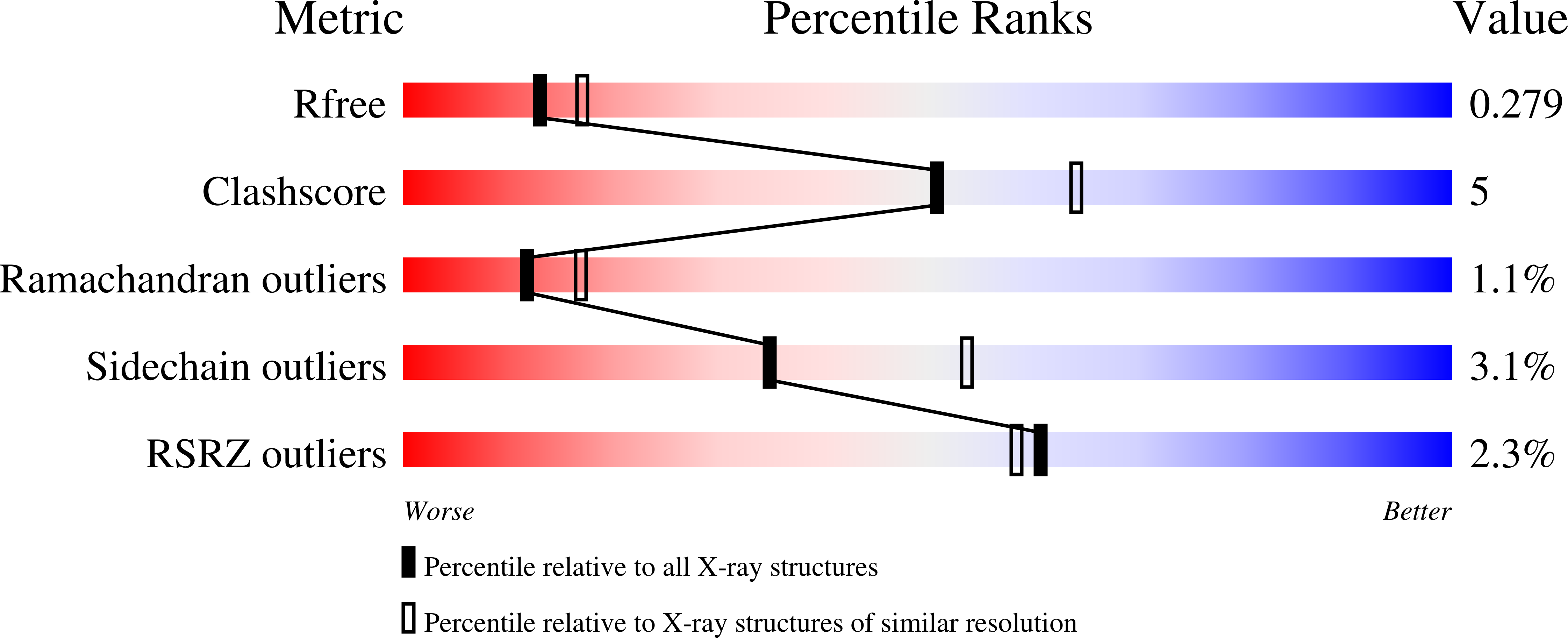
Resolution:
2.42 Å
R-Value Free:
0.27
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
P 1