Deposition Date
2024-11-08
Release Date
2025-07-23
Last Version Date
2025-08-06
Entry Detail
PDB ID:
9E9J
Keywords:
Title:
L-allo-threonine aldolase from Thermotoga maritima, N308E-Y87A-R122G-P121D Mutant
Biological Source:
Source Organism:
Thermotoga maritima (Taxon ID: 2336)
Host Organism:
Method Details:
Experimental Method:
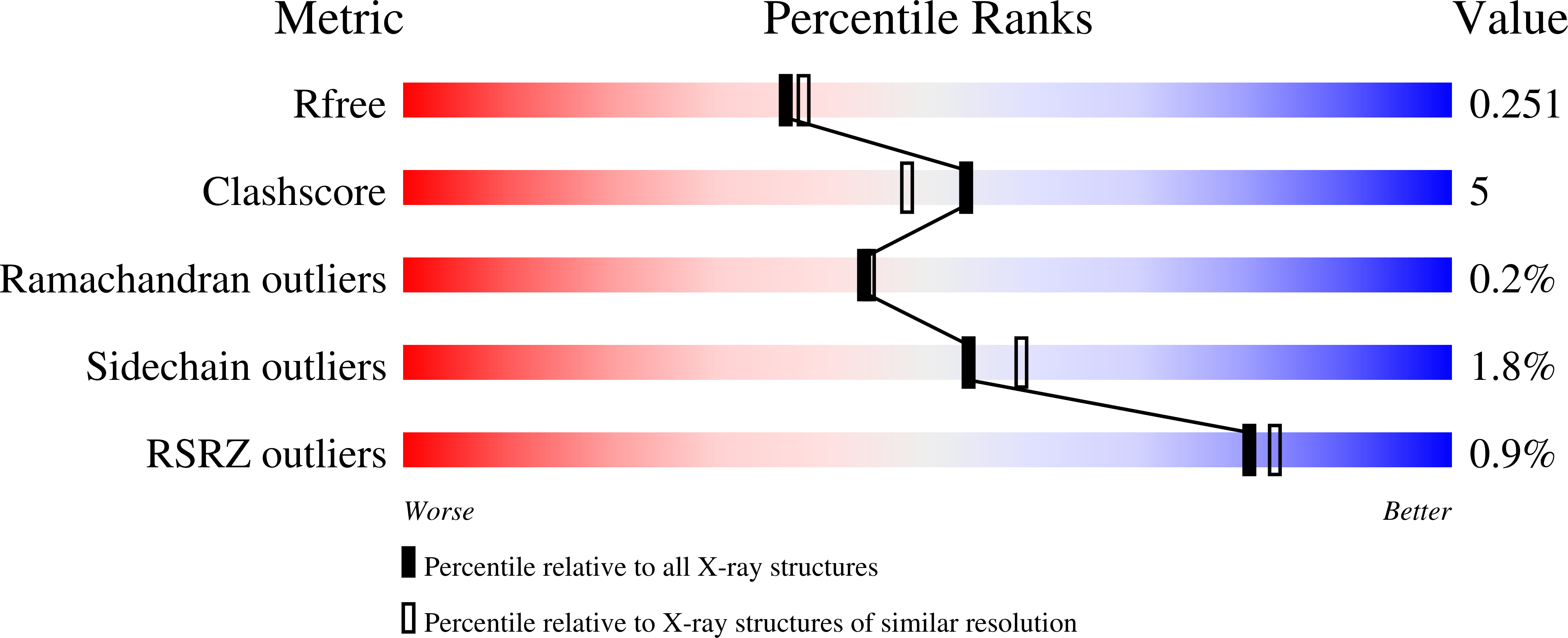
Resolution:
2.15 Å
R-Value Free:
0.25
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 21 21 21