Deposition Date
2024-11-01
Release Date
2025-05-07
Last Version Date
2025-07-23
Entry Detail
PDB ID:
9E7J
Keywords:
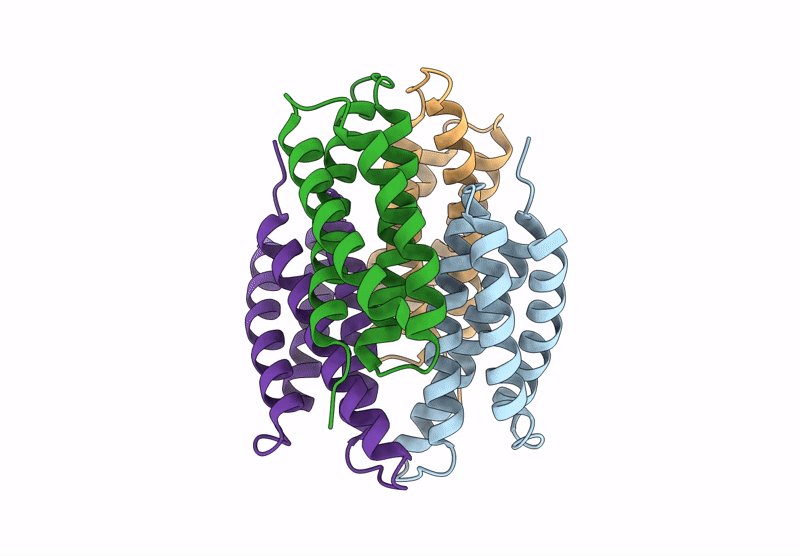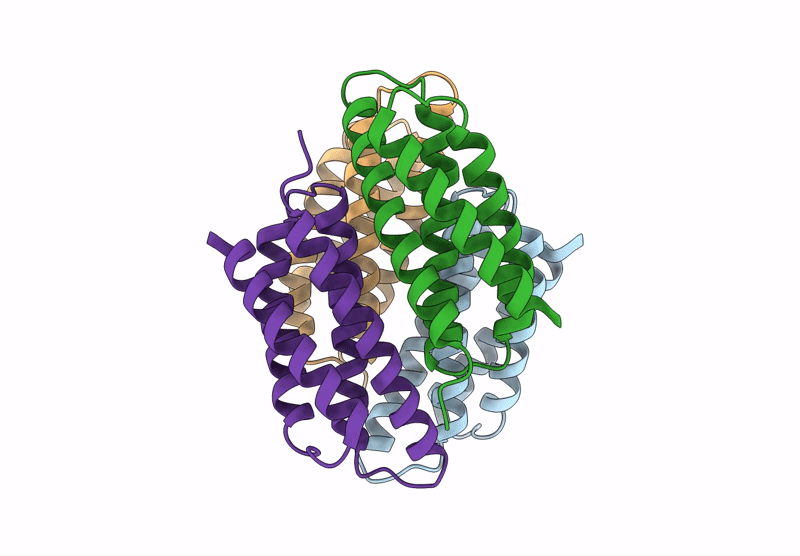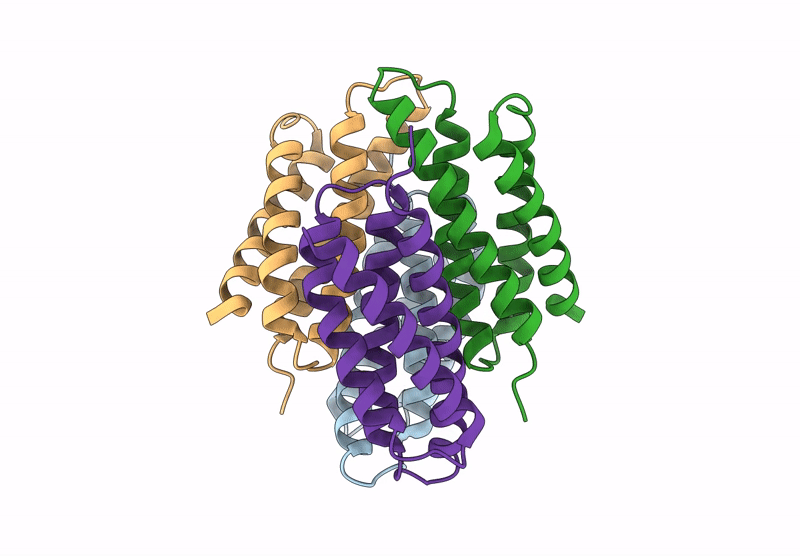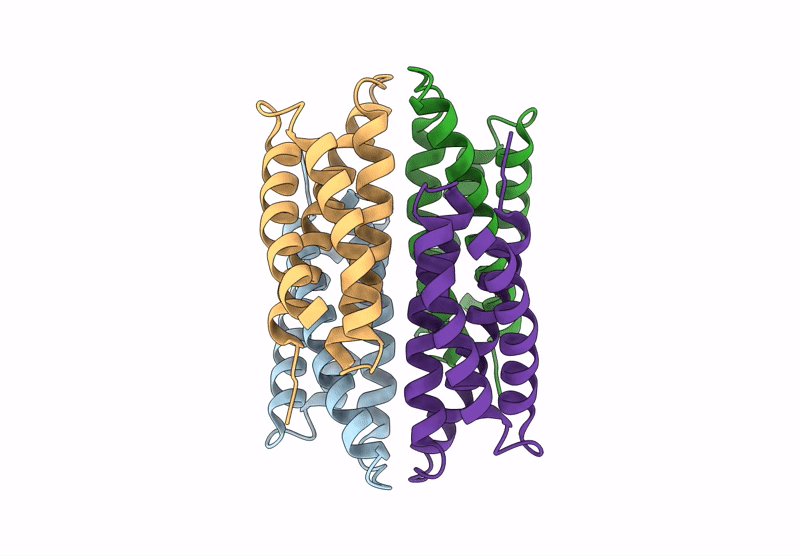
Title:
Cu-bound tetrameric copper storage protein 1 with anti-rhodopsin 1D4 epitope
Biological Source:
Source Organism:
Methylosinus trichosporium OB3b (Taxon ID: 595536)
Host Organism:
Method Details:
Experimental Method:
Resolution:
2.98 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


