Abstact
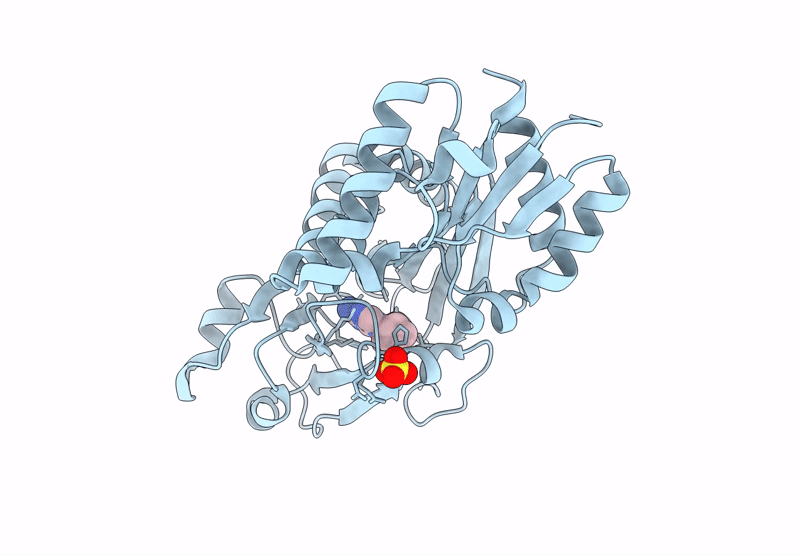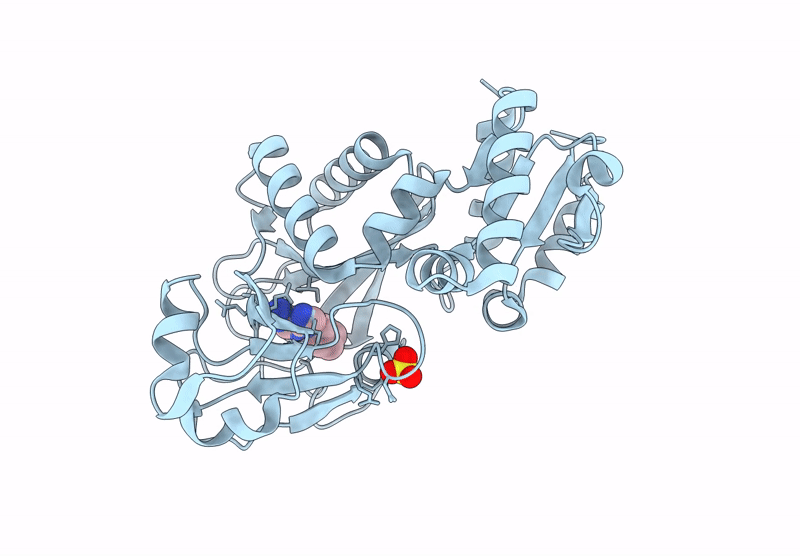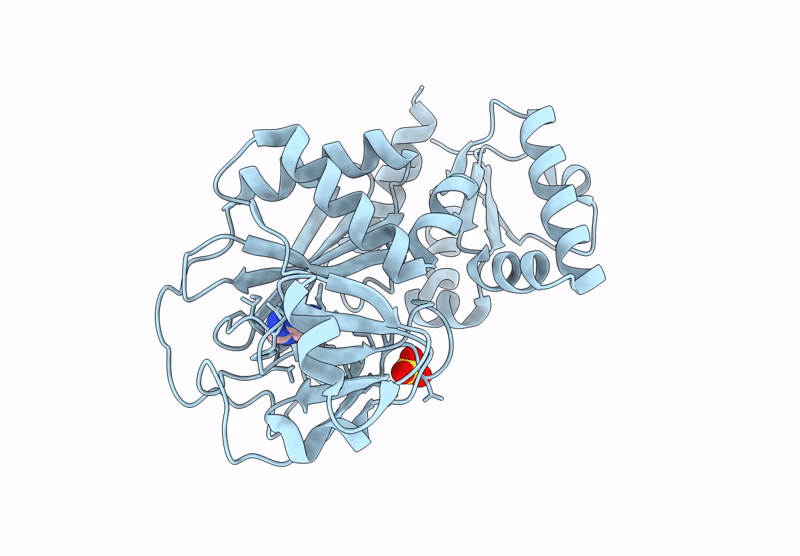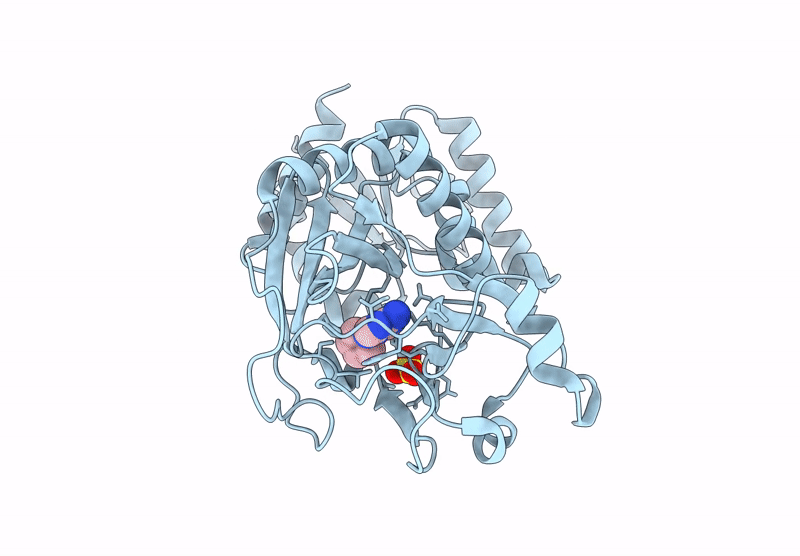
Inositol phosphates (IPs) and inositol pyrophosphate play critical roles in many biological processes as signaling molecules in pathways responsible for cellular functions involved in growth and maintenance. The biosynthesis of IPs is carried out by a family of inositol phosphate kinases. In mammals, Inositol tetrakisphosphate kinase-1 (ITPK1) phosphorylates inositol-1,3,4-trisphosphate (Ins(1,3,4)P3) and inositol-3,4,5,6-tetrakisphosphate (IP4), generating inositol-1,3,4,5,6-pentakisphosphate (IP5), which can be further phosphorylated to become inositol hexakisphosphate (IP6). ITPK1 also possesses phosphatase activity that can convert IP5 back to IP4; therefore, ITPK1 may serve as a regulatory step in IP6 production. IP6 utilization has been implicated in processes fundamental to cellular sustainability that are severely perturbed in many disease states including RNA editing, DNA repair, chromatin structure organization, and ubiquitin ligation. Therefore, ITPK1, with no known inhibitors in the literature, is a potential molecular target for modulating important processes in several human diseases. By independently coupling ITPK1 phosphatase and kinase activities to luciferase activity, we have developed and used biochemical high throughput assays to discover eight ITPK1 inhibitors. Further analysis revealed that three of these leads inhibit ITPK1 in an ATP-competitive manner, with low micromolar to nanomolar affinities. We further demonstrate that the most potent ITPK1 inhibitor can regulate cellular ITPK1 activity. We determined the crystal structure of ITPK1 in complex with this inhibitor at a resolution of 2.25 Å. This work provides insight into the design of potential next-generation inhibitors.