Deposition Date
2024-08-22
Release Date
2025-02-19
Last Version Date
2025-04-02
Entry Detail
PDB ID:
9DAK
Keywords:
Title:
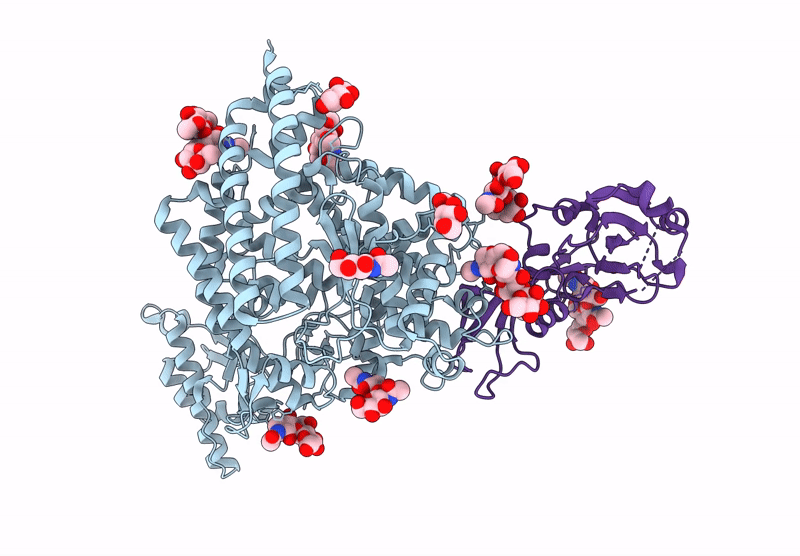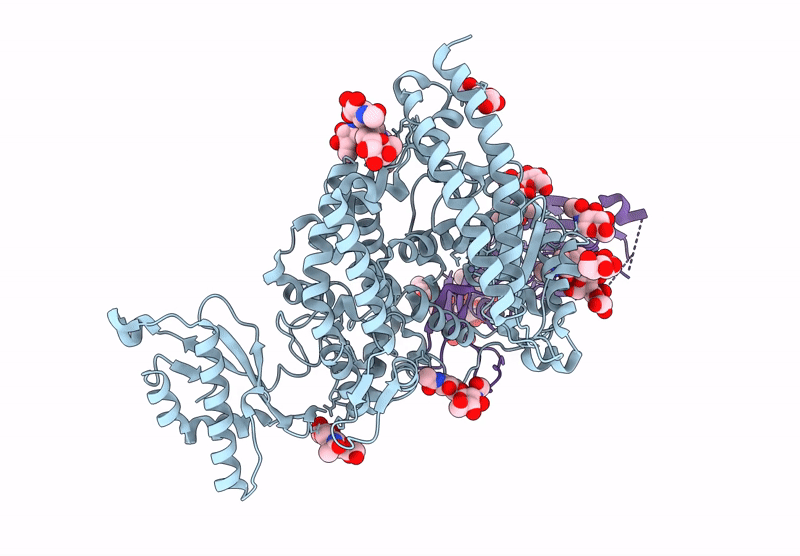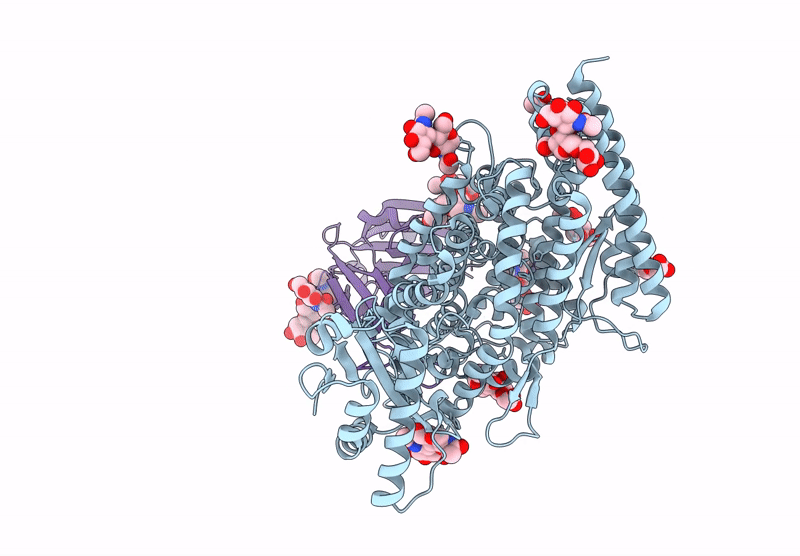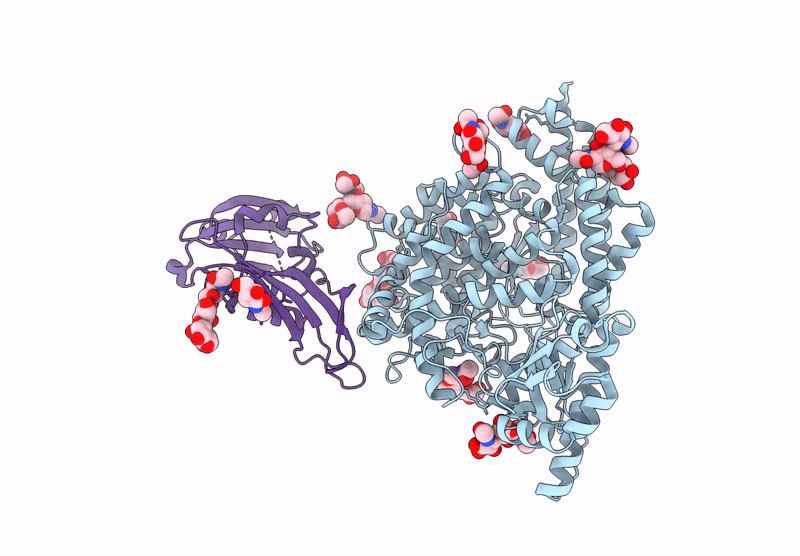
Merbecovirus PnNL2018B Spike glycoprotein RBD bound to the P. Nathusii ACE2
Biological Source:
Source Organism(s):
Pipistrellus nathusii (Taxon ID: 59473)
Merbecovirus (Taxon ID: 2509494)
Merbecovirus (Taxon ID: 2509494)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.40 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


