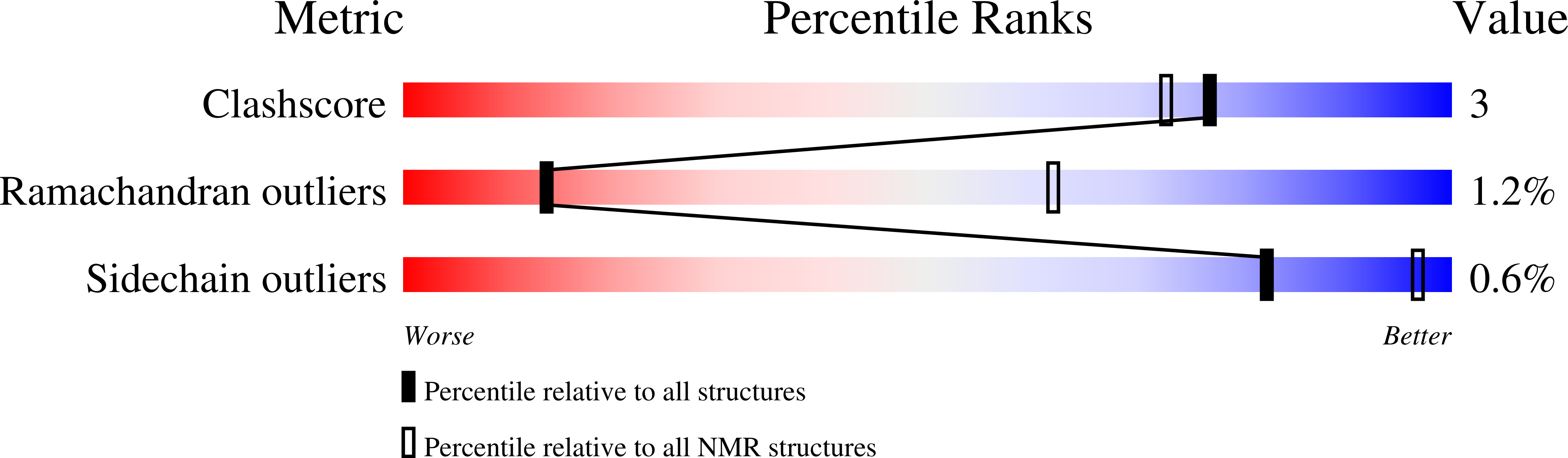
Deposition Date
2024-08-21
Release Date
2025-04-02
Last Version Date
2025-04-02
Entry Detail
PDB ID:
9D9B
Keywords:
Title:
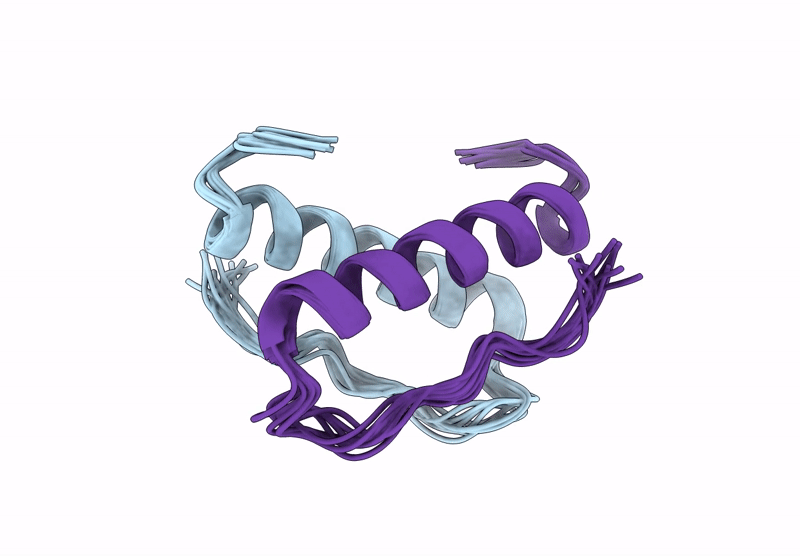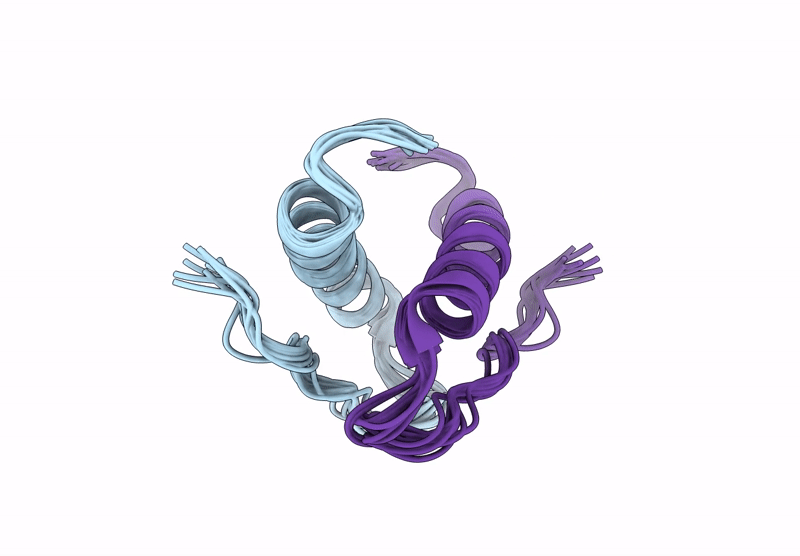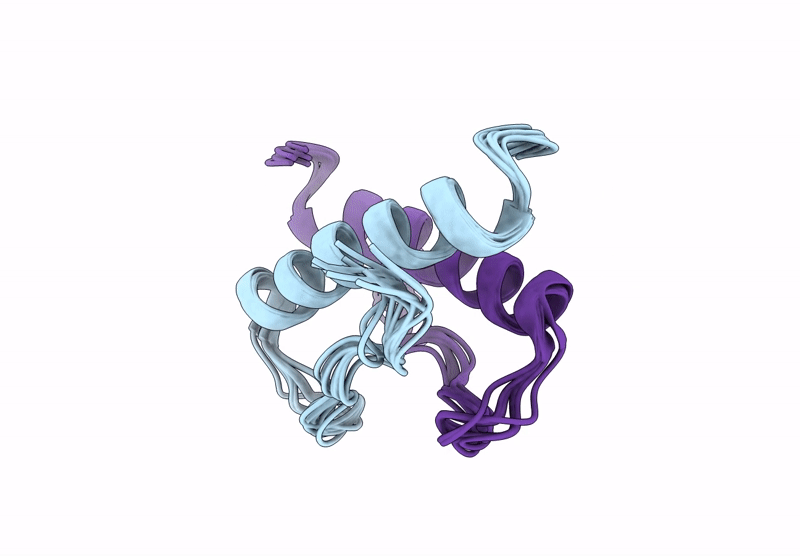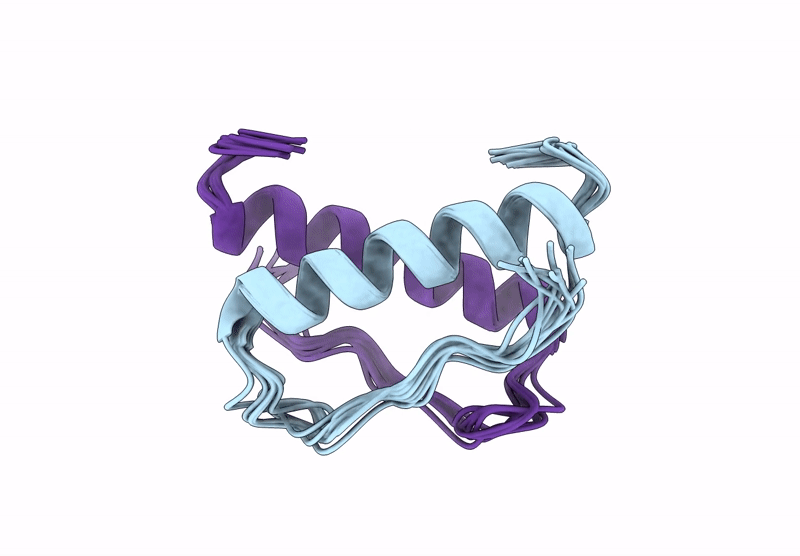
Incorporation of dehydro-aza-proline residues in avian pancreatic polypeptide: Aza-Pro substitution at position 6
Biological Source:
Source Organism(s):
Meleagris gallopavo (Taxon ID: 9103)
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
10
Selection Criteria:
structures with the lowest energy