Deposition Date
2024-08-13
Release Date
2025-09-03
Last Version Date
2025-09-03
Entry Detail
PDB ID:
9D5L
Keywords:
Title:
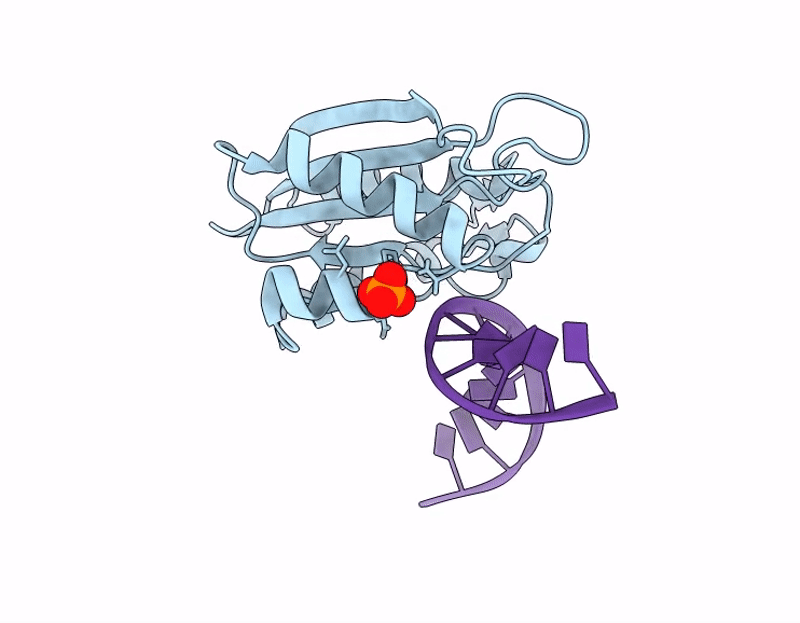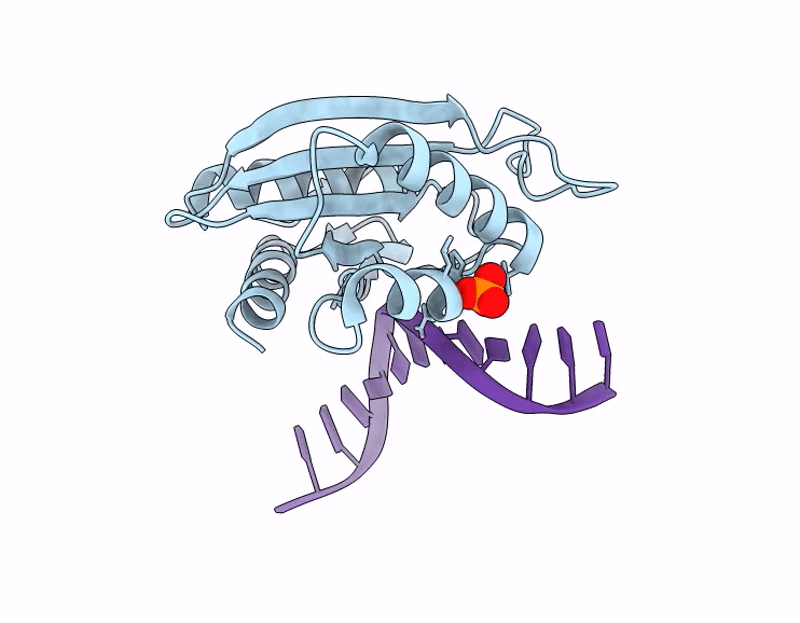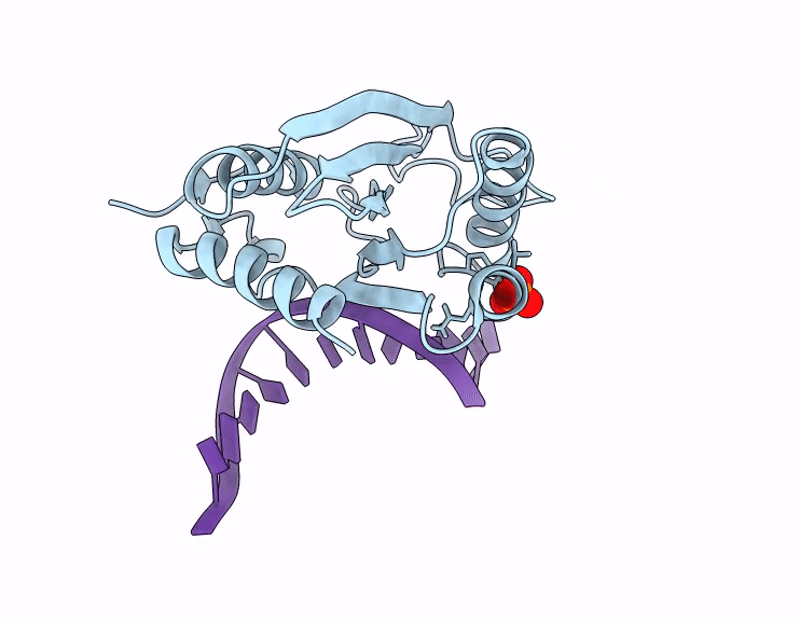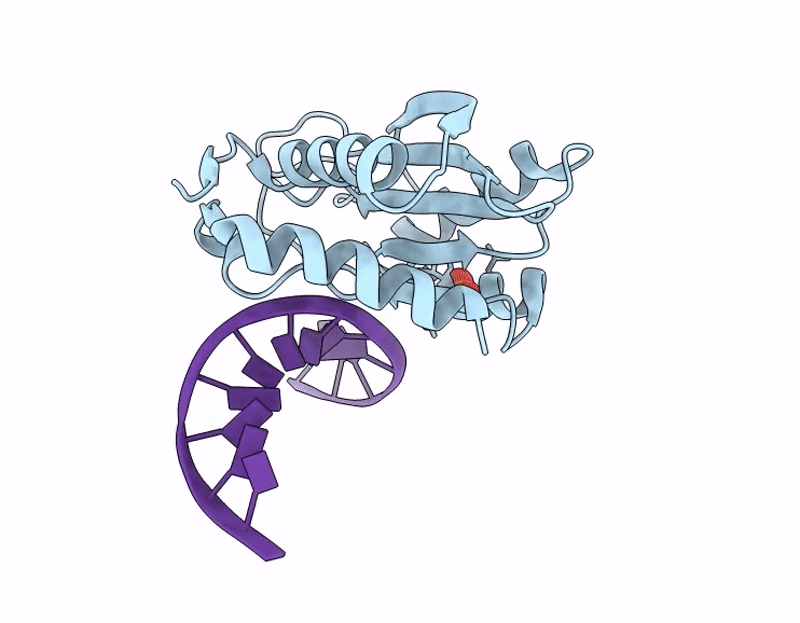
The C-terminal domain of Thiopseudomonas alkaliphila Tn7 TnsE bound to DNA
Biological Source:
Source Organism(s):
Thiopseudomonas alkaliphila (Taxon ID: 1697053)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
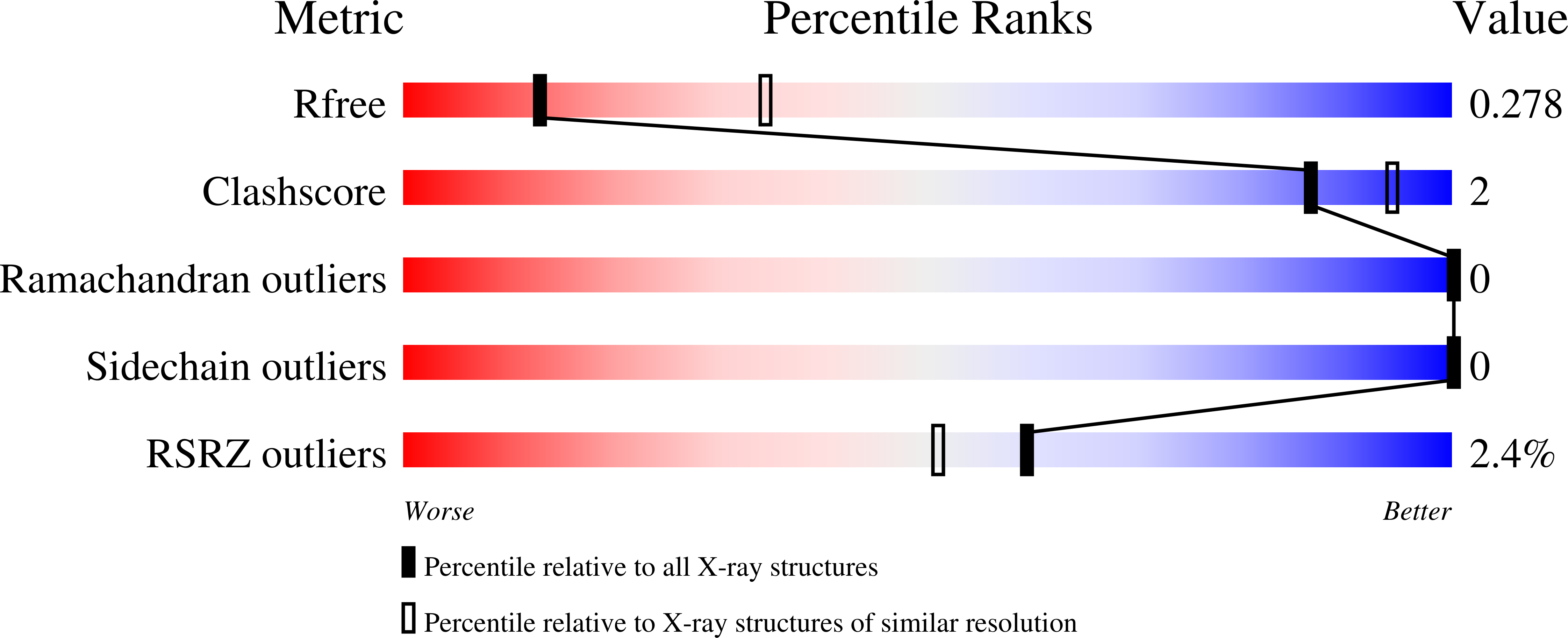
Resolution:
2.80 Å
R-Value Free:
0.27
R-Value Work:
0.23
R-Value Observed:
0.24
Space Group:
P 61 2 2