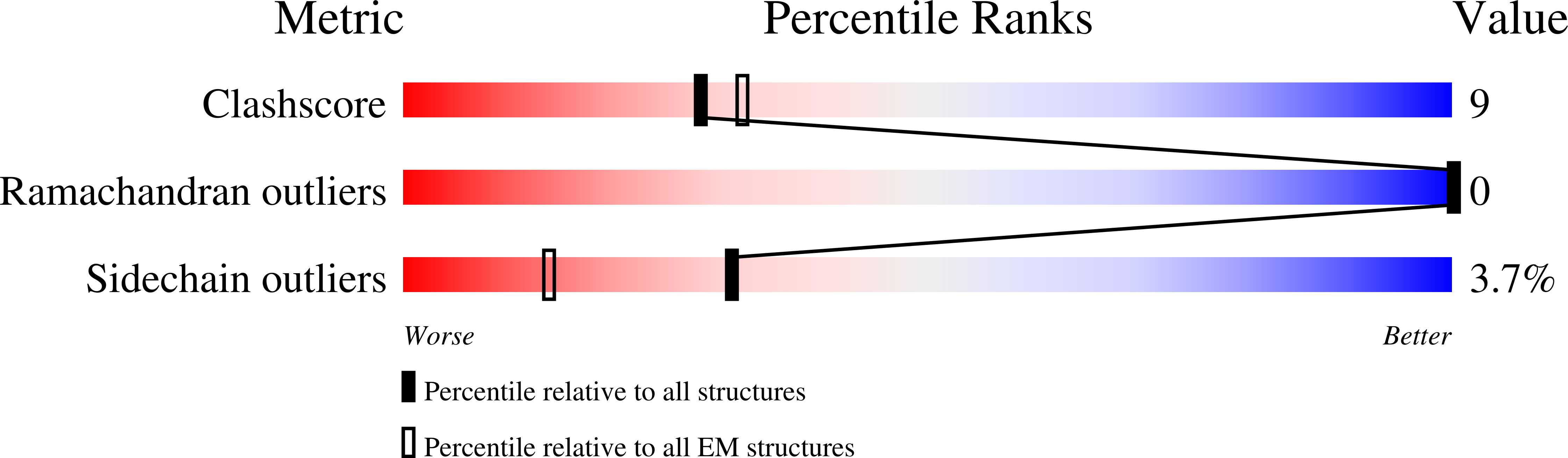Deposition Date
2024-05-23
Release Date
2025-03-19
Last Version Date
2025-03-26
Entry Detail
PDB ID:
9BXX
Keywords:
Title:
Class 11 model for pre-reduction condition of Bacillus subtilis ribonucleotide reductase complex
Biological Source:
Source Organism(s):
Bacillus subtilis (Taxon ID: 1423)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
4.26 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE