Deposition Date
2024-05-16
Release Date
2025-04-23
Last Version Date
2025-10-29
Entry Detail
PDB ID:
9BUB
Keywords:
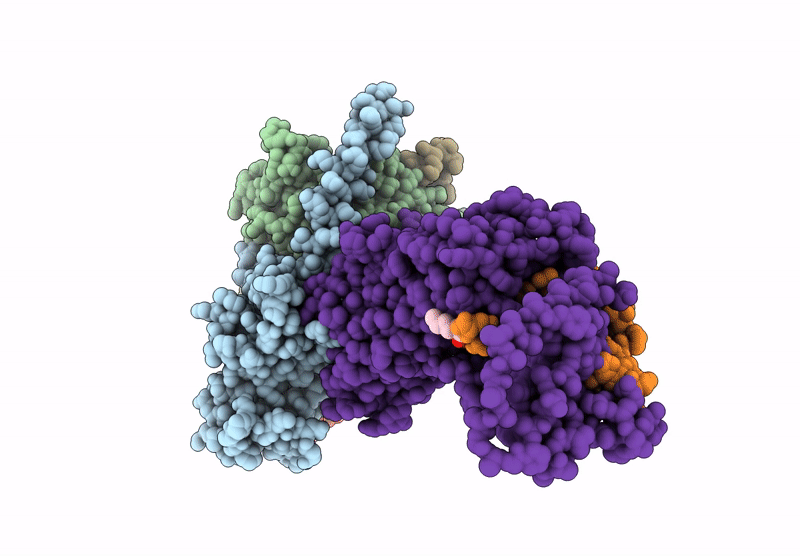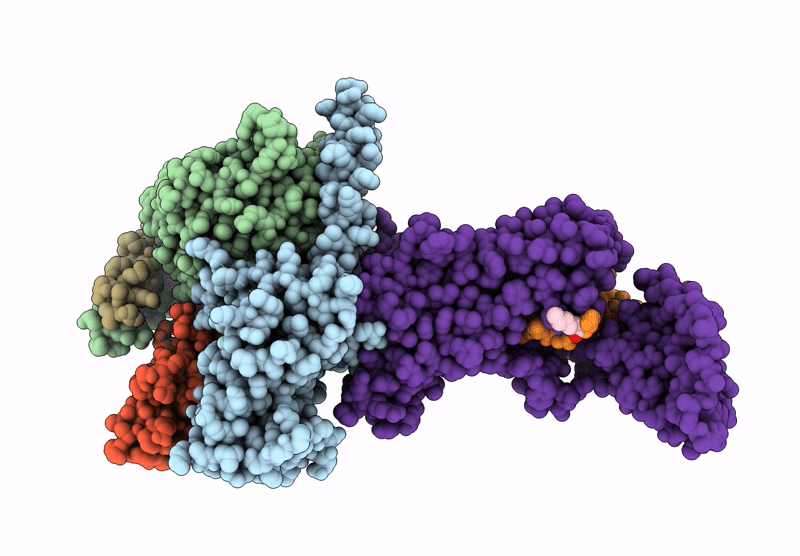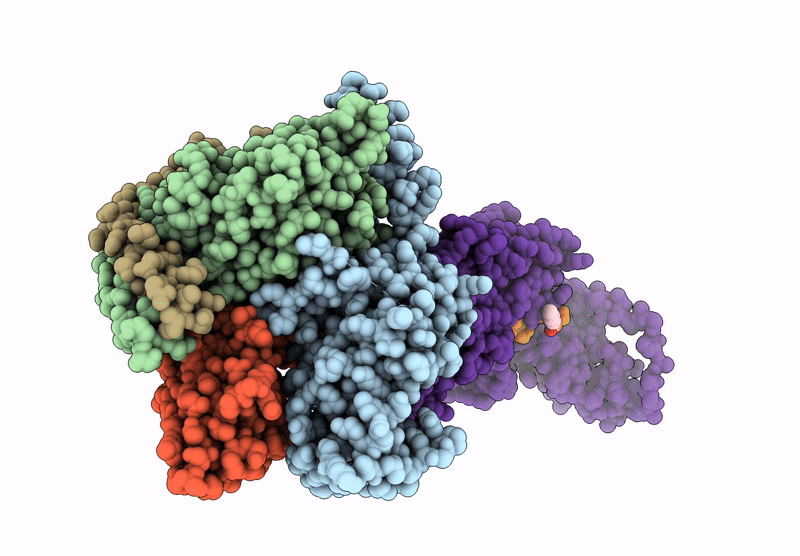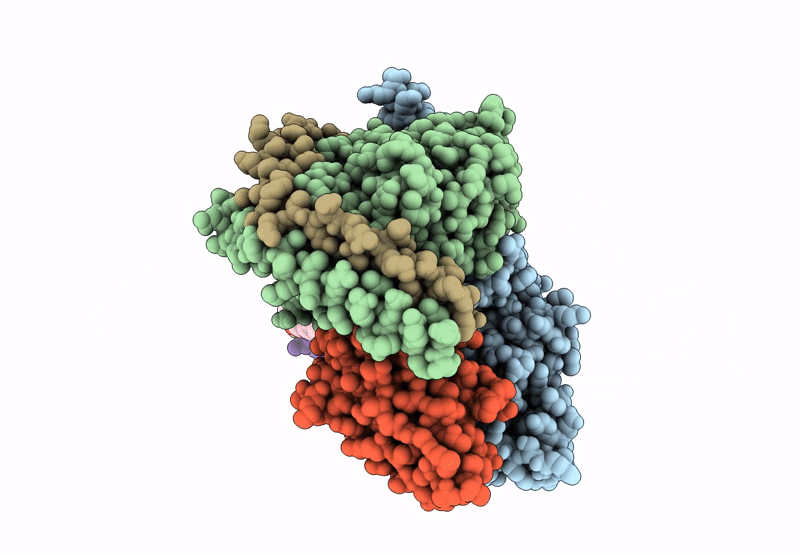
Title:
Human calcitonin Receptor in complex with Gs and cagrilintide in the bypass conformation
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Lama glama (Taxon ID: 9844)
Lama glama (Taxon ID: 9844)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.30 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


