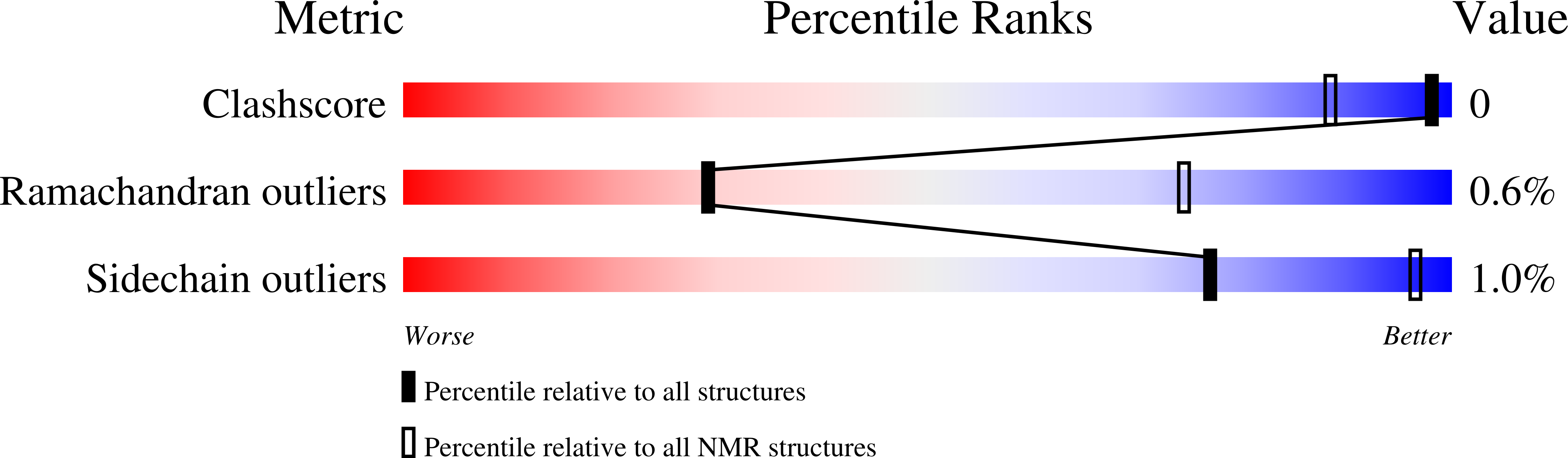
Deposition Date
2024-04-18
Release Date
2024-06-05
Last Version Date
2024-11-20
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
20
Selection Criteria:
structures with acceptable covalent geometry