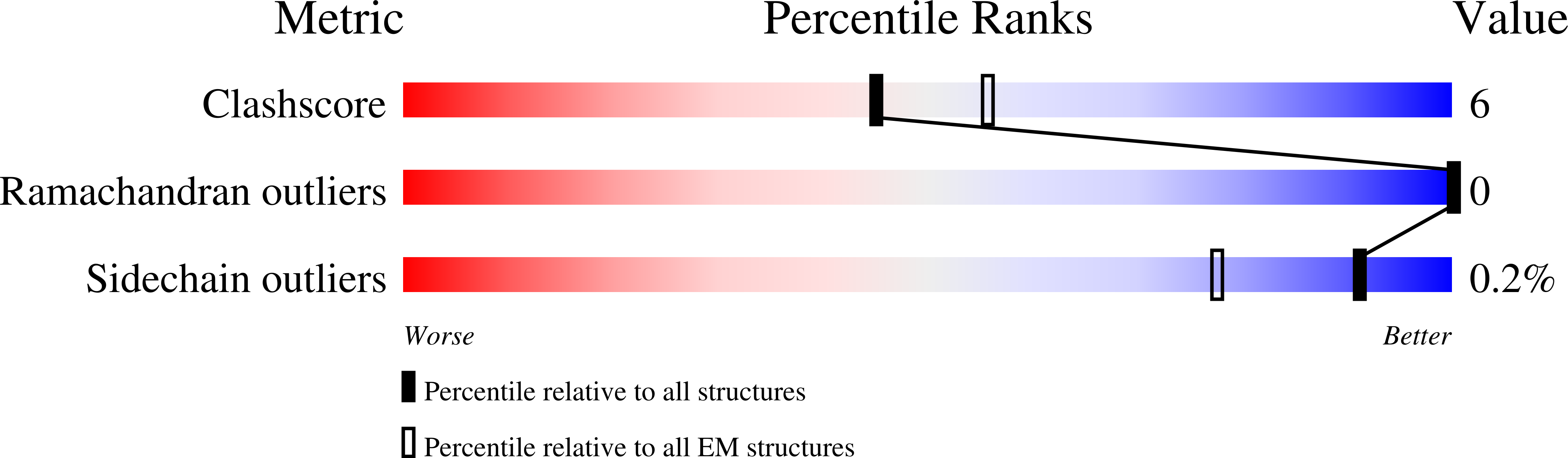Deposition Date
2024-04-03
Release Date
2024-11-20
Last Version Date
2025-05-28
Entry Detail
PDB ID:
9BA0
Keywords:
Title:
Structural mechanism of CB1R binding to peripheral and biased inverse agonists
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Pyrococcus abyssi GE5 (Taxon ID: 272844)
Lama glama (Taxon ID: 9844)
Pyrococcus abyssi GE5 (Taxon ID: 272844)
Lama glama (Taxon ID: 9844)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.13 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE