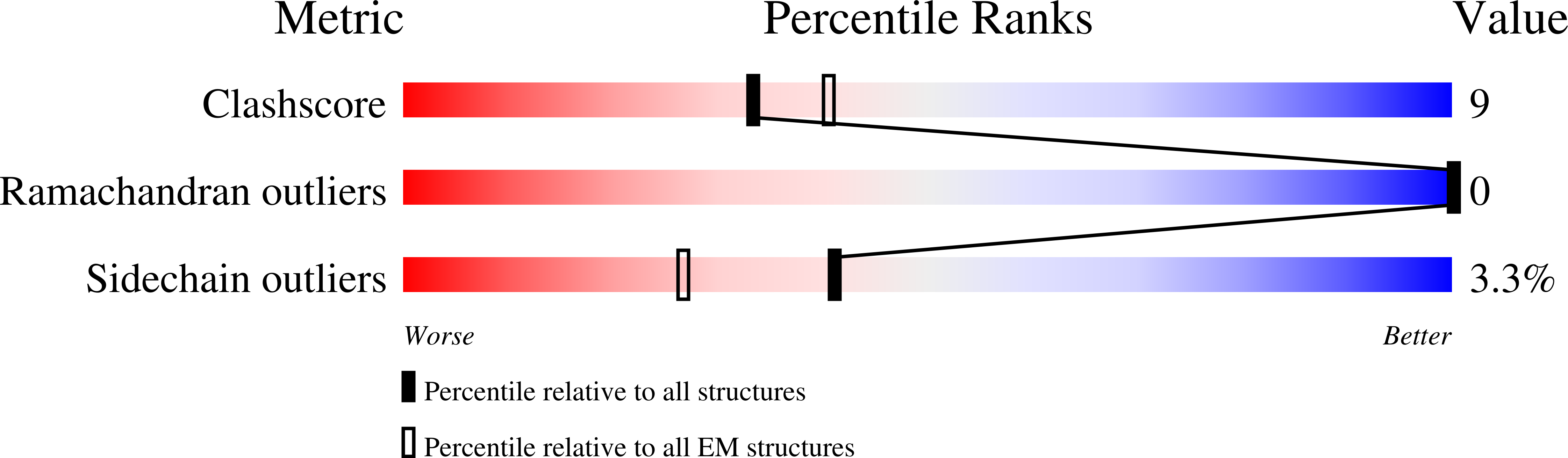
Deposition Date
2024-03-23
Release Date
2024-07-31
Last Version Date
2024-11-06
Entry Detail
PDB ID:
9B64
Keywords:
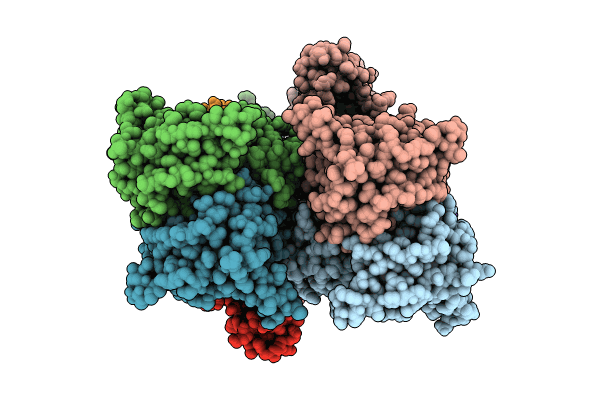
Title:
GluA2 flip Q in complex with TARPgamma2 at pH5, class23, structure of LBD-TMD-TARPgamma2
Biological Source:
Source Organism(s):
Mus musculus (Taxon ID: 10090)
Rattus norvegicus (Taxon ID: 10116)
Rattus norvegicus (Taxon ID: 10116)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.56 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE