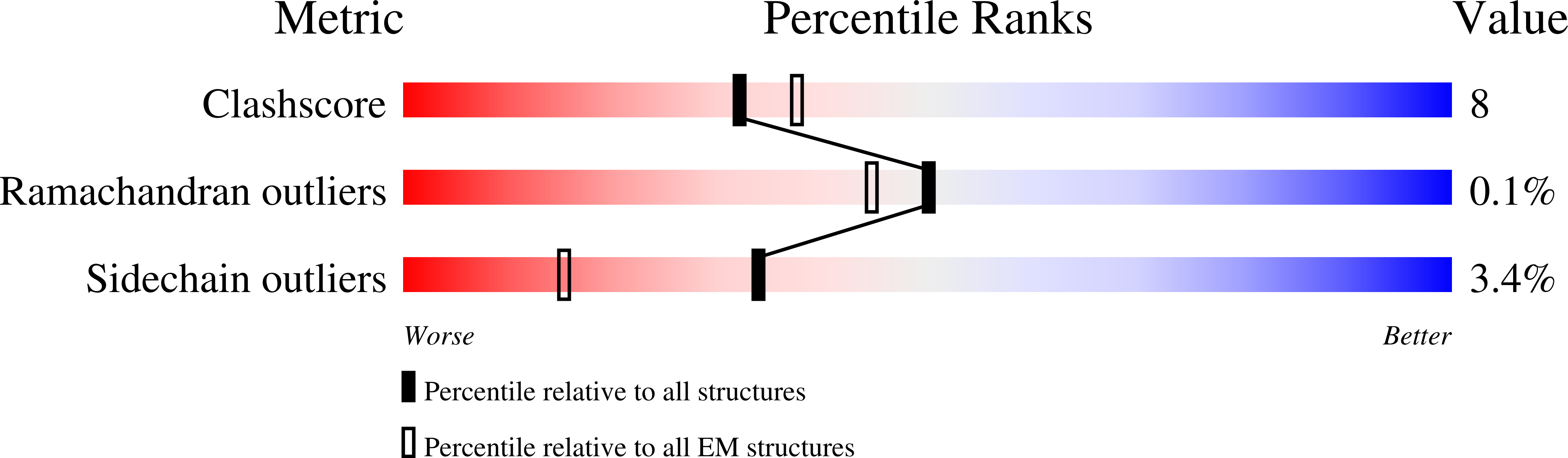
Deposition Date
2024-02-23
Release Date
2025-01-22
Last Version Date
2025-05-28
Entry Detail
PDB ID:
9ARK
Keywords:
Title:
CryoEM structure of BoNT-NTNH-OrfX2 complex from Clostridium botulinum E1, minor class
Biological Source:
Source Organism(s):
Clostridium botulinum E1 str. 'BoNT E Beluga' (Taxon ID: 536233)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
4.10 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE