Deposition Date
2024-03-28
Release Date
2024-12-18
Last Version Date
2024-12-18
Entry Detail
PDB ID:
8YVJ
Keywords:
Title:
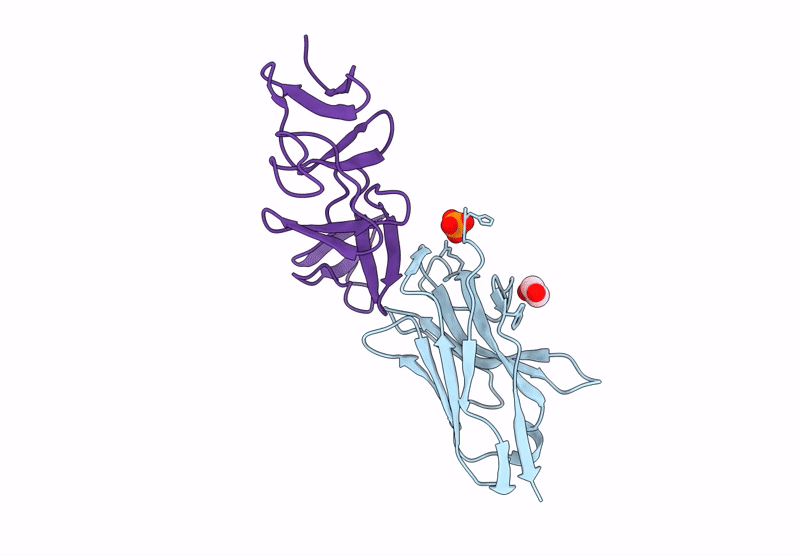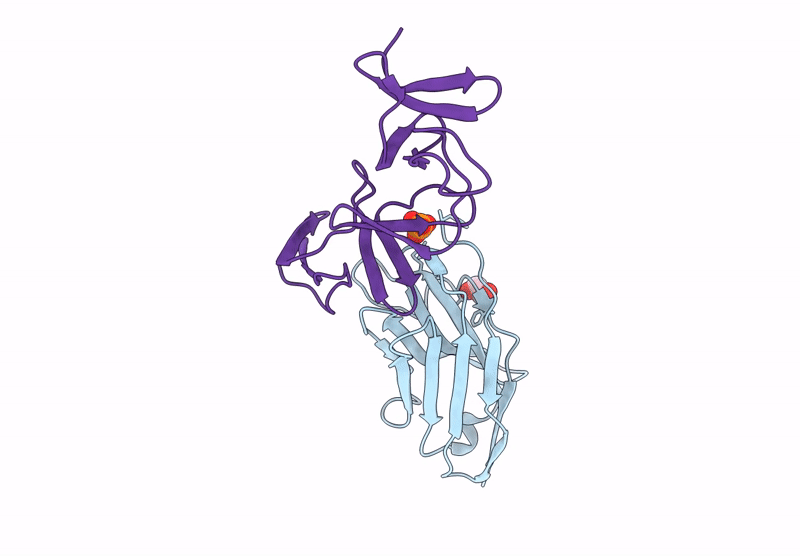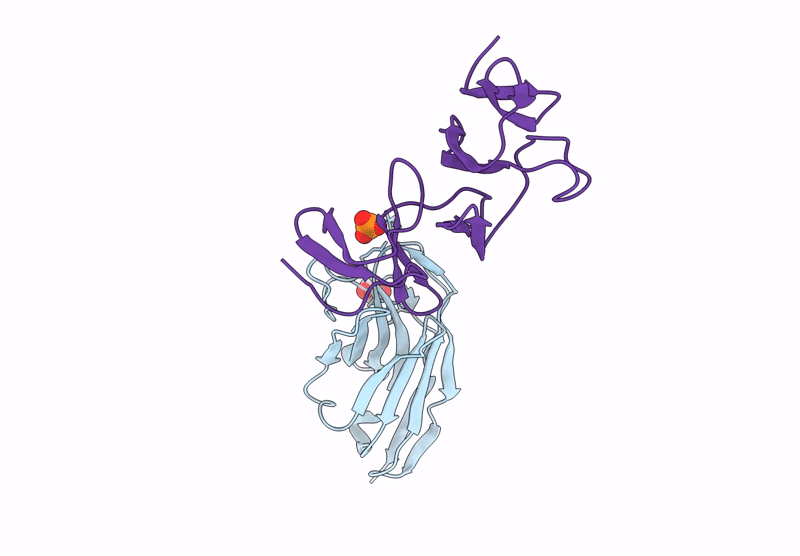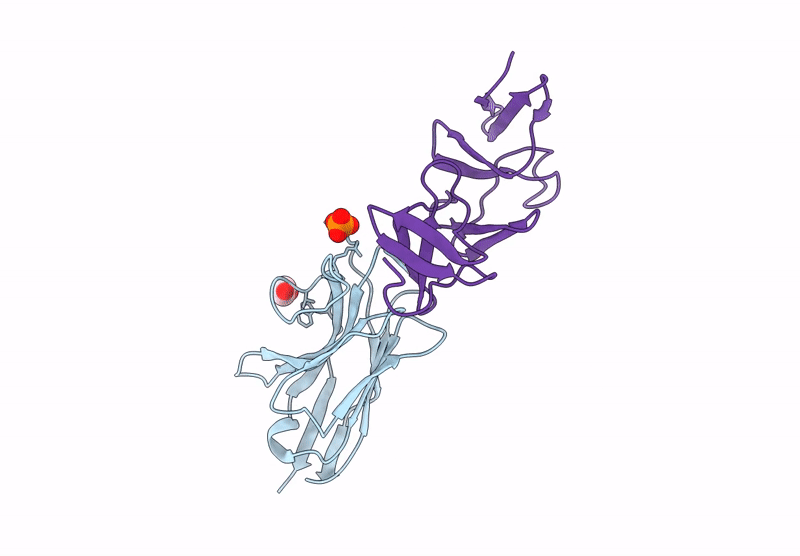
Crystal structure of the C. difficile toxin A CROPs domain fragment 2592-2710 bound to H5.2 nanobody
Biological Source:
Source Organism(s):
Camelus dromedarius (Taxon ID: 9838)
Clostridioides difficile 342 (Taxon ID: 1151306)
Clostridioides difficile 342 (Taxon ID: 1151306)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
1.65 Å
R-Value Free:
0.19
R-Value Work:
0.14
R-Value Observed:
0.15
Space Group:
P 21 21 21


