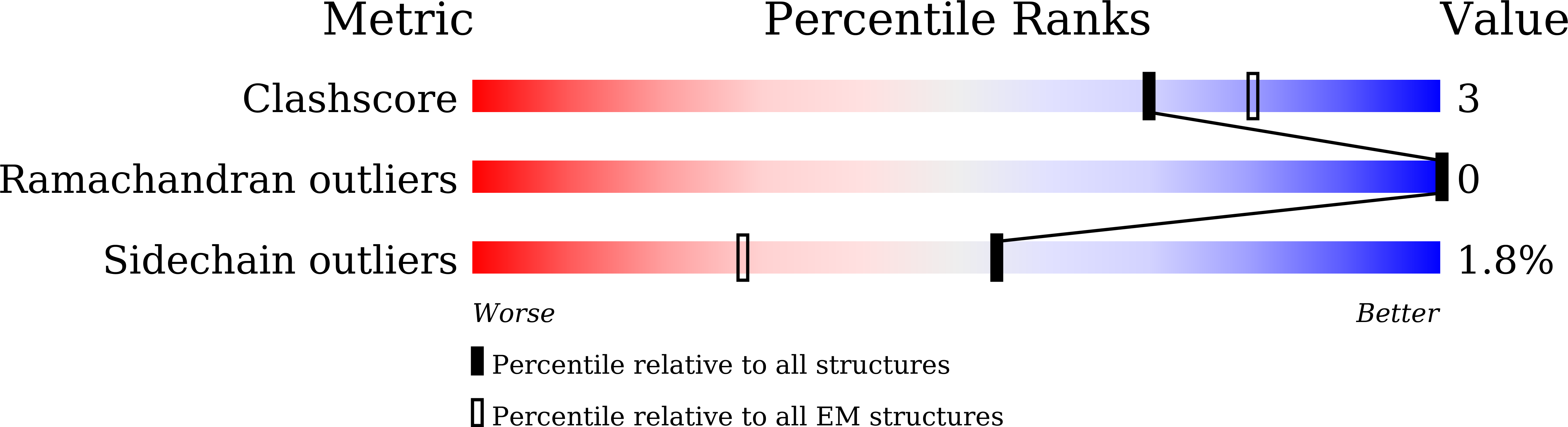
Deposition Date
2024-03-28
Release Date
2024-12-11
Last Version Date
2024-12-11
Entry Detail
PDB ID:
8YVC
Keywords:
Title:
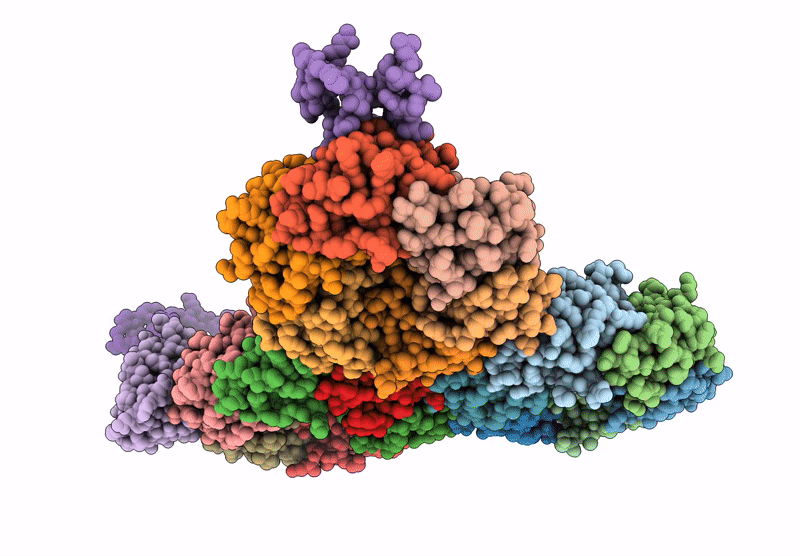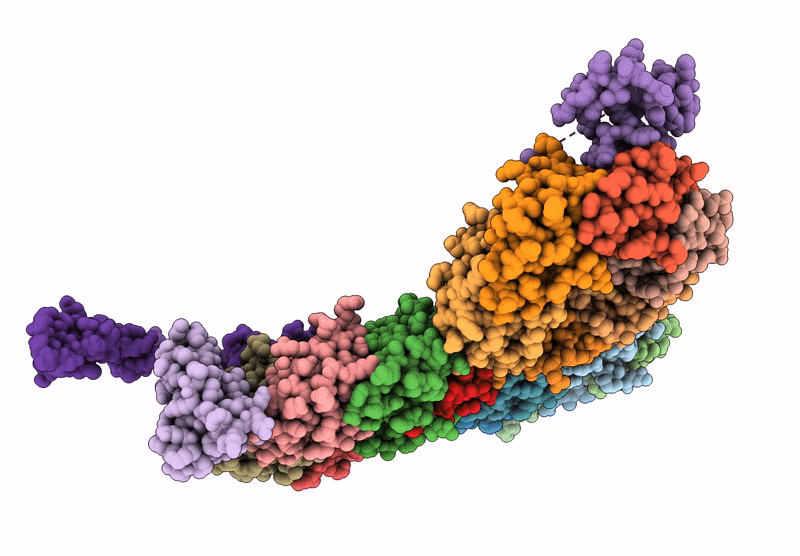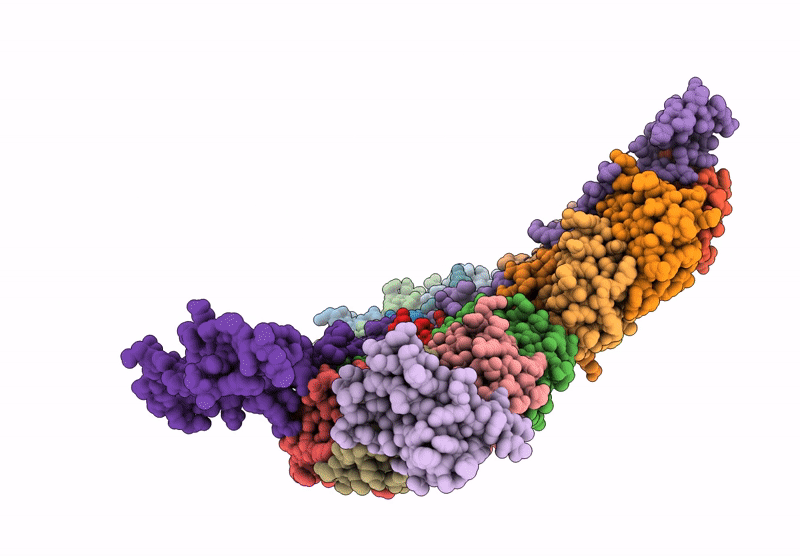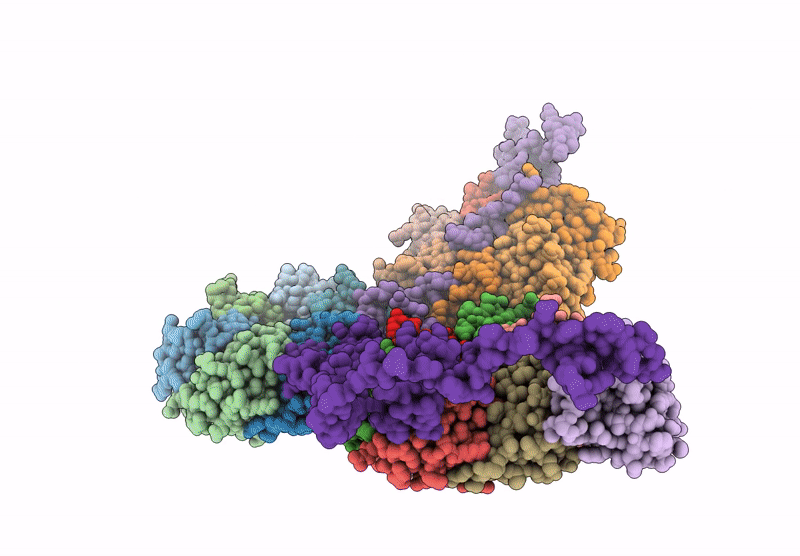
Cryo-EM structure of carboxysomal midi-shell:icosahedral assembly from CsoS4A/4B/1A/1B/1C/1D and CsoS2 C-terminal co-expression (T = 19)
Biological Source:
Source Organism(s):
Halothiobacillus neapolitanus (Taxon ID: 927)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.04 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE