Deposition Date
2024-02-11
Release Date
2025-02-12
Last Version Date
2025-04-02
Entry Detail
PDB ID:
8YB3
Keywords:
Title:
XFEL crystal structure of the oxygen-bound form of F87A/F393H P450BM3 with N-enanthyl-L-prolyl-L-phenylalanine in complex with styrene
Biological Source:
Source Organism(s):
Priestia megaterium NBRC 15308 = ATCC 14581 (Taxon ID: 1348623)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
1.50 Å
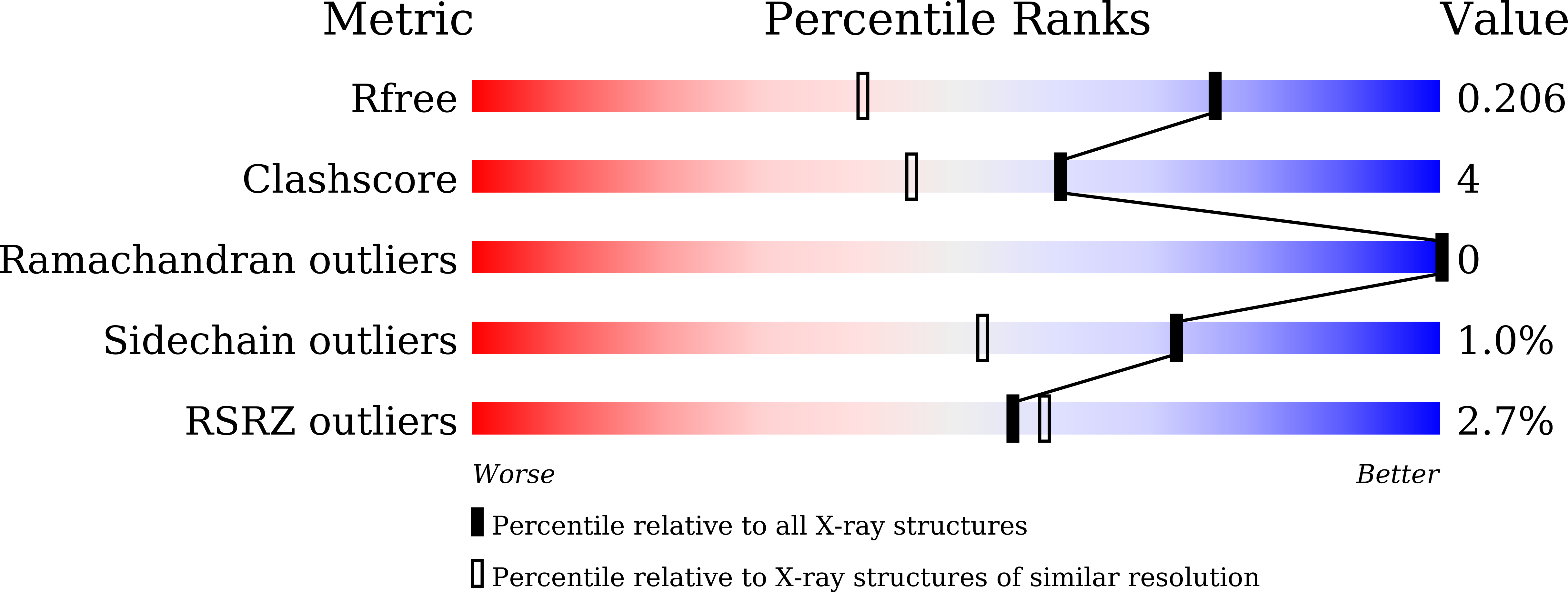
R-Value Free:
0.20
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 21 21 21