Deposition Date
2024-02-03
Release Date
2024-07-10
Last Version Date
2025-01-15
Entry Detail
PDB ID:
8Y74
Keywords:
Title:
Crystal structure of 9-mer peptide from H9N2 avian influenza virus in complex with BF2*0201
Biological Source:
Source Organism(s):
Gallus gallus (Taxon ID: 9031)
Influenza A virus (A/chicken/Bangladesh/19495/2013(H9N2)) (Taxon ID: 1482006)
Influenza A virus (A/chicken/Bangladesh/19495/2013(H9N2)) (Taxon ID: 1482006)
Expression System(s):
Method Details:
Experimental Method:
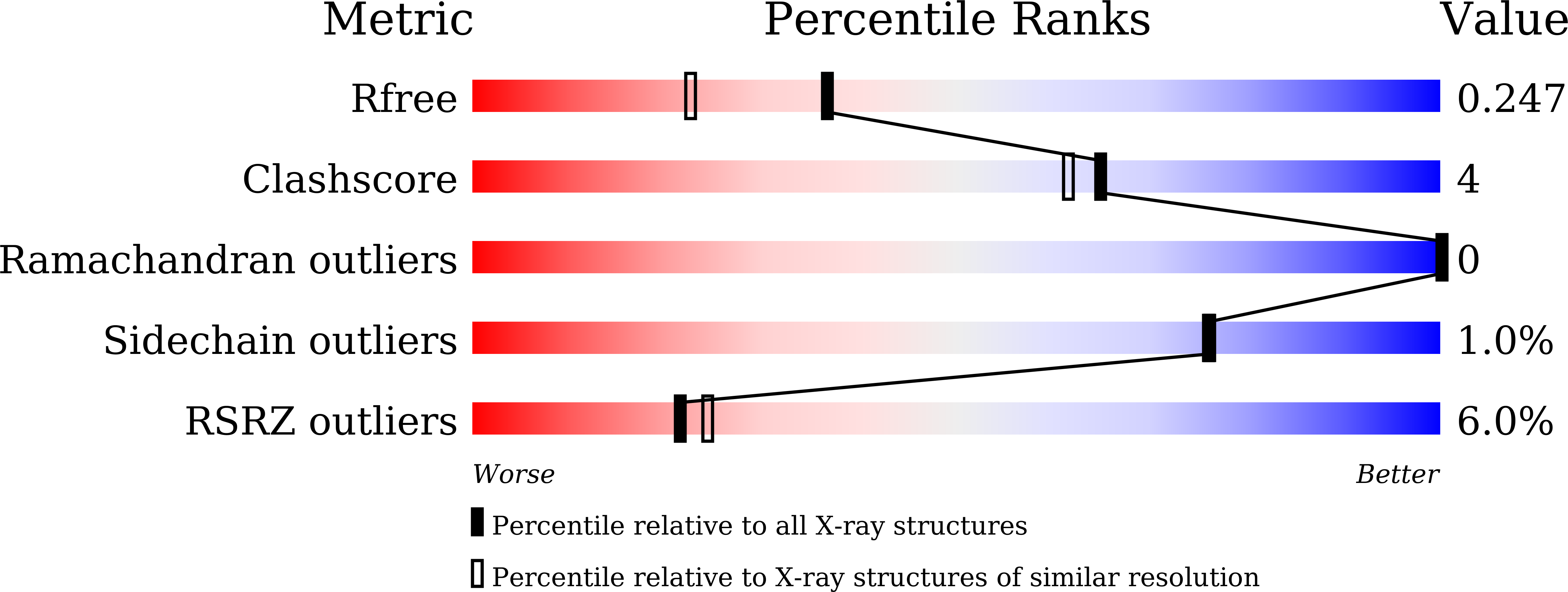
Resolution:
1.90 Å
R-Value Free:
0.24
R-Value Work:
0.20
Space Group:
C 1 2 1