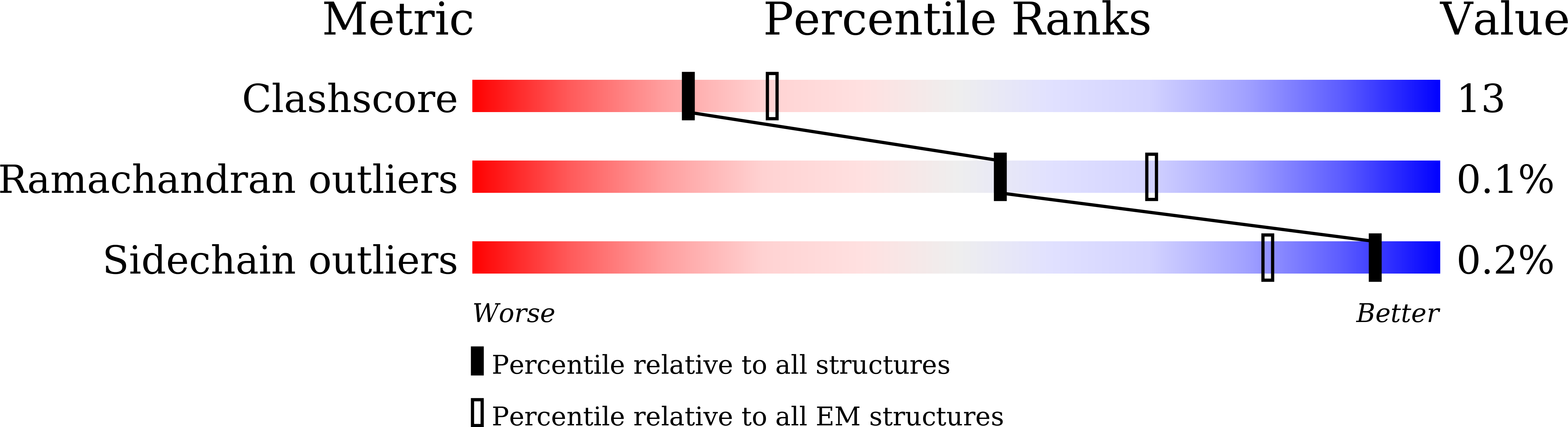
Deposition Date
2024-01-08
Release Date
2024-04-10
Last Version Date
2024-05-08
Entry Detail
PDB ID:
8XS0
Keywords:
Title:
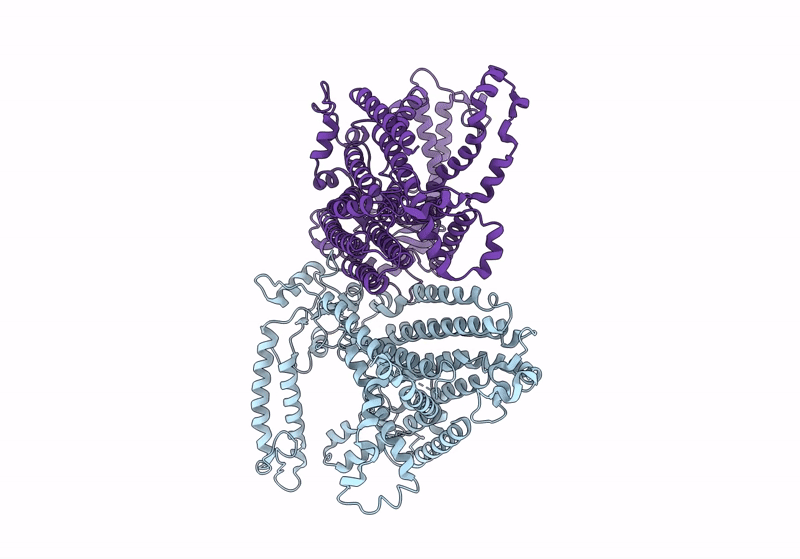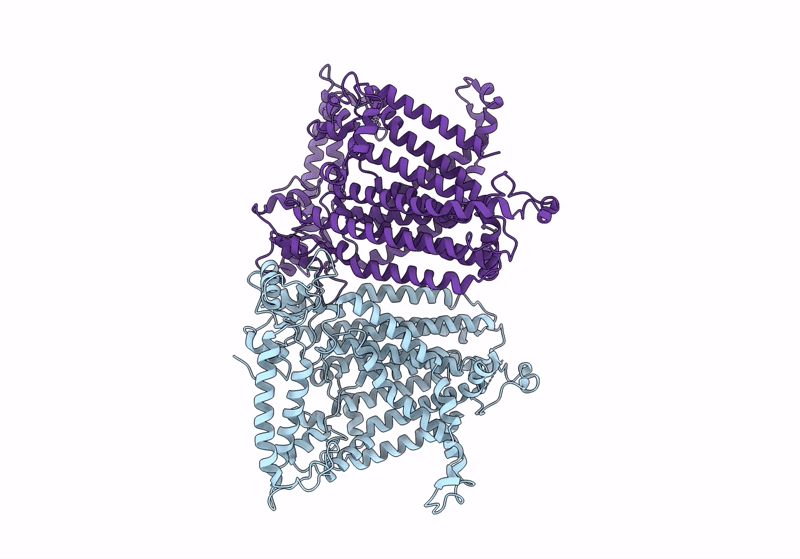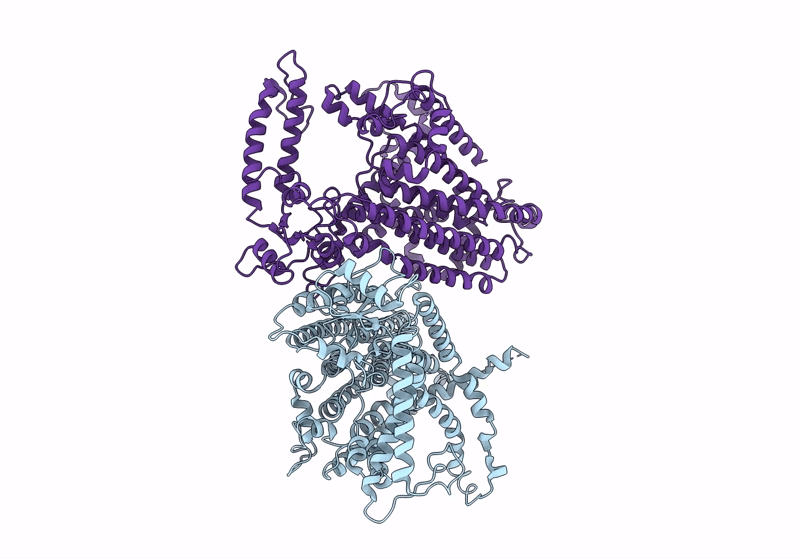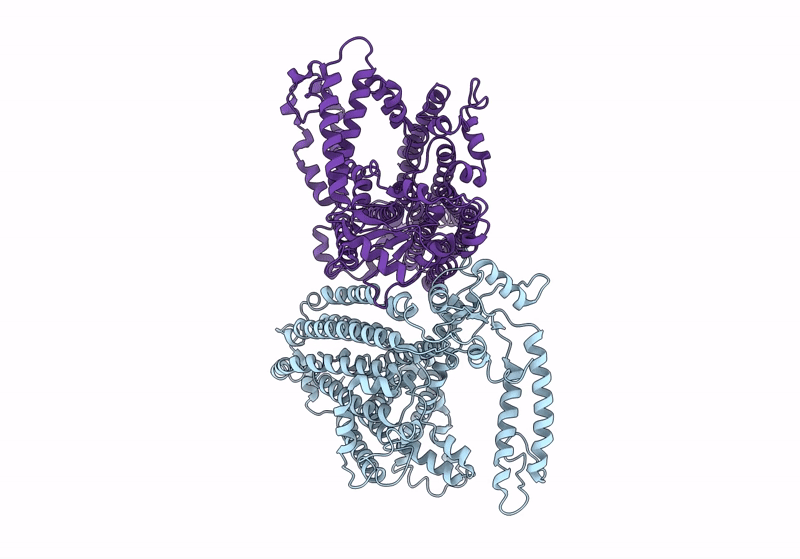
Cryo-EM structure of OSCA3.1-1.1ver(Y367N-G454S-Y458I)-open/'desensitized' state
Biological Source:
Source Organism(s):
Arabidopsis thaliana (Taxon ID: 3702)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.89 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE