Deposition Date
1992-04-01
Release Date
1993-07-15
Last Version Date
2024-02-14
Entry Detail
PDB ID:
8XIM
Keywords:
Title:
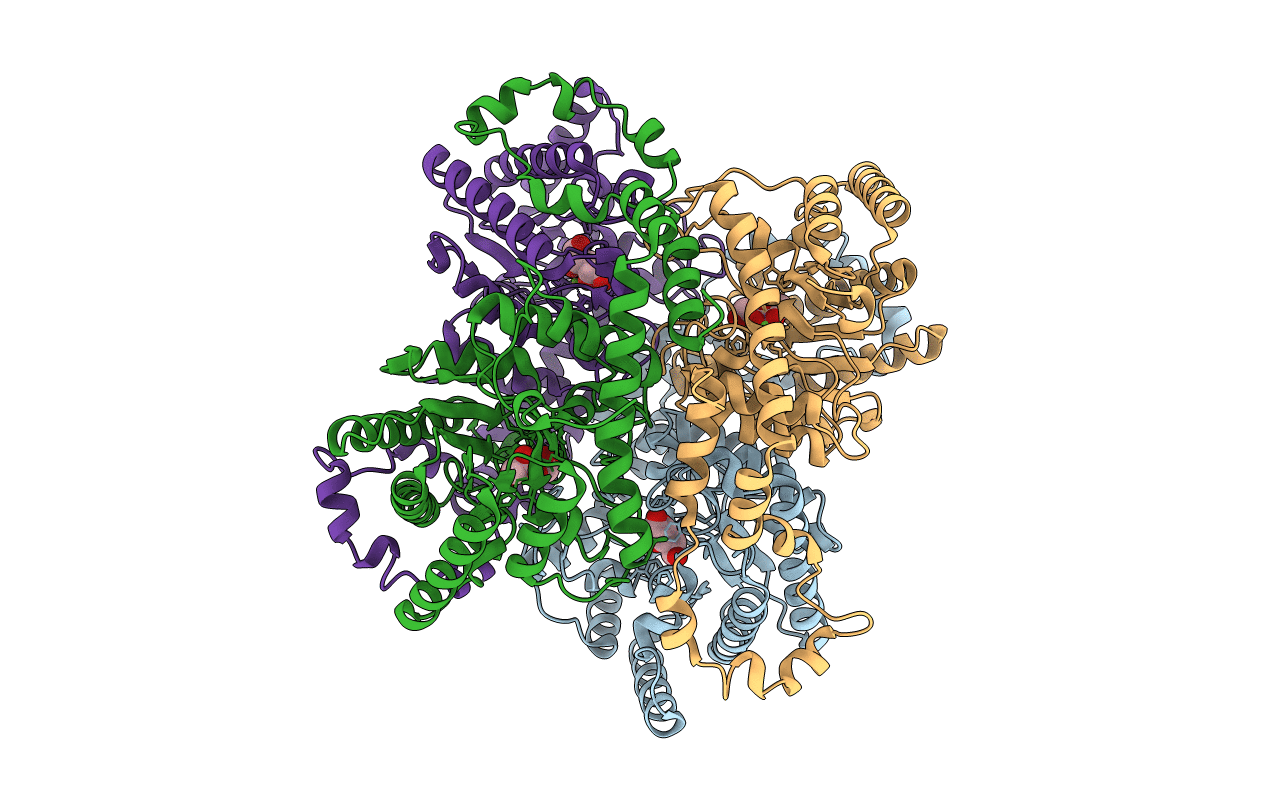
PROTEIN ENGINEERING OF XYLOSE (GLUCOSE) ISOMERASE FROM ACTINOPLANES MISSOURIENSIS. 1. CRYSTALLOGRAPHY AND SITE-DIRECTED MUTAGENESIS OF METAL BINDING SITES
Biological Source:
Source Organism(s):
Actinoplanes missouriensis (Taxon ID: 1866)
Method Details:
Experimental Method:
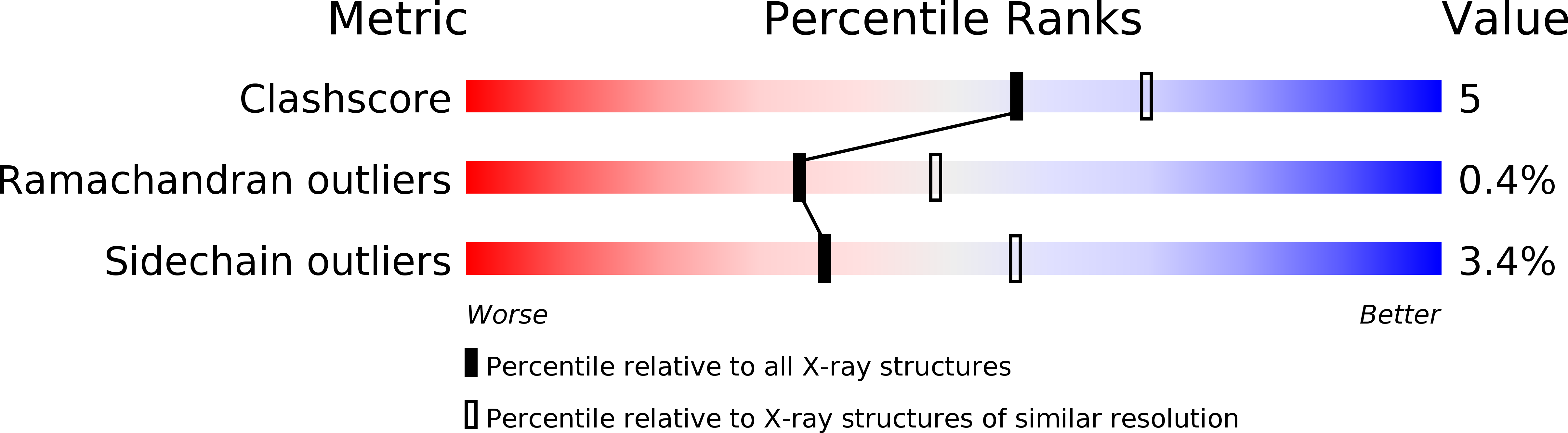
Resolution:
2.40 Å
R-Value Observed:
0.14
Space Group:
P 32 2 1