Deposition Date
2023-11-21
Release Date
2024-11-27
Last Version Date
2025-10-15
Entry Detail
PDB ID:
8X6K
Keywords:
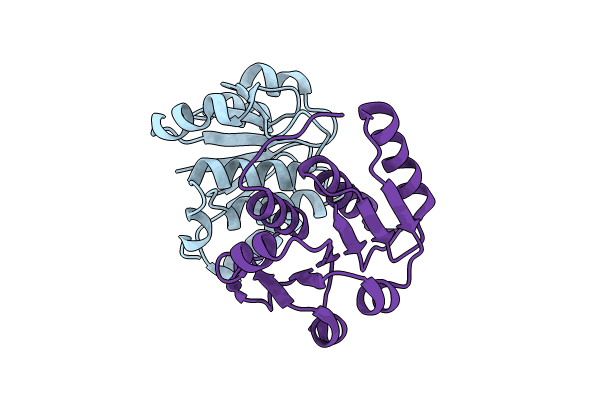
Title:
The X-ray structure of N-terminal catalytic domain of Thermoplasma acidophilum tRNA methyltransferase Trm56 (Ta0931).
Biological Source:
Source Organism(s):
Thermoplasma acidophilum DSM 1728 (Taxon ID: 273075)
Expression System(s):
Method Details:
Experimental Method:
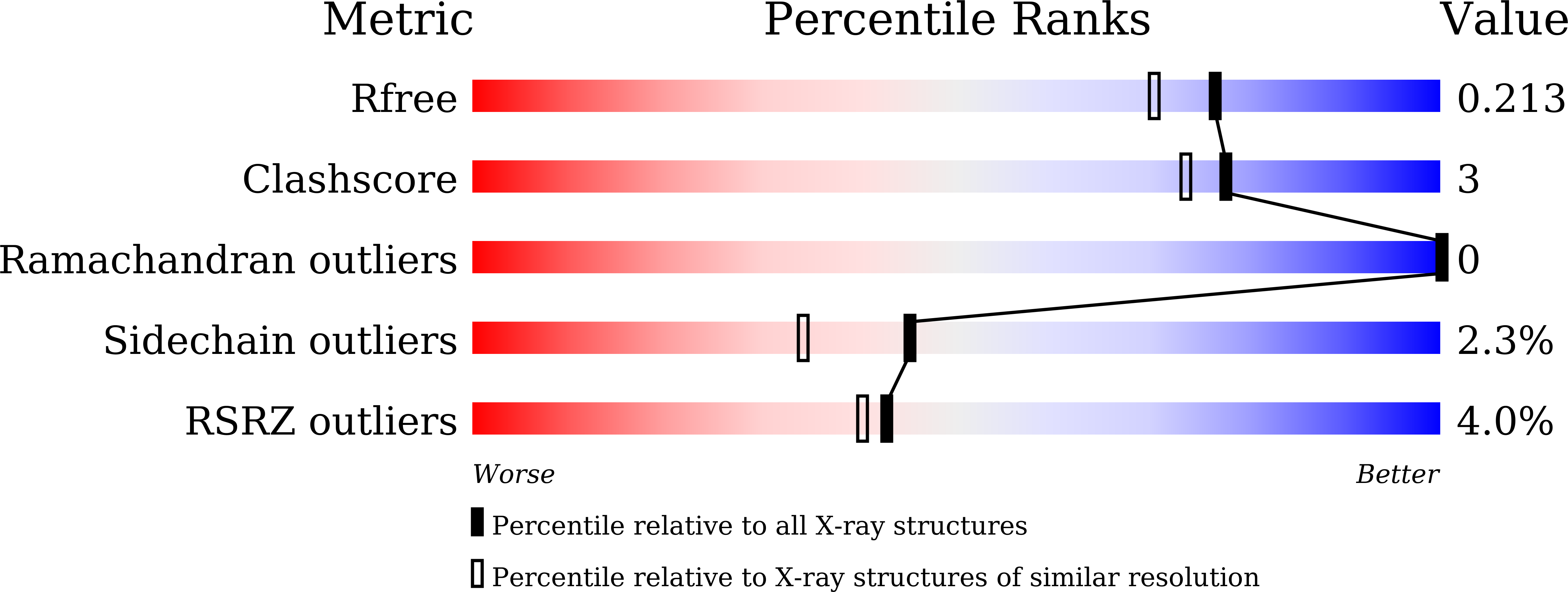
Resolution:
1.80 Å
R-Value Free:
0.20
R-Value Work:
0.16
Space Group:
P 32 2 1