Deposition Date
2023-11-16
Release Date
2024-02-28
Last Version Date
2024-10-16
Entry Detail
PDB ID:
8X51
Keywords:
Title:
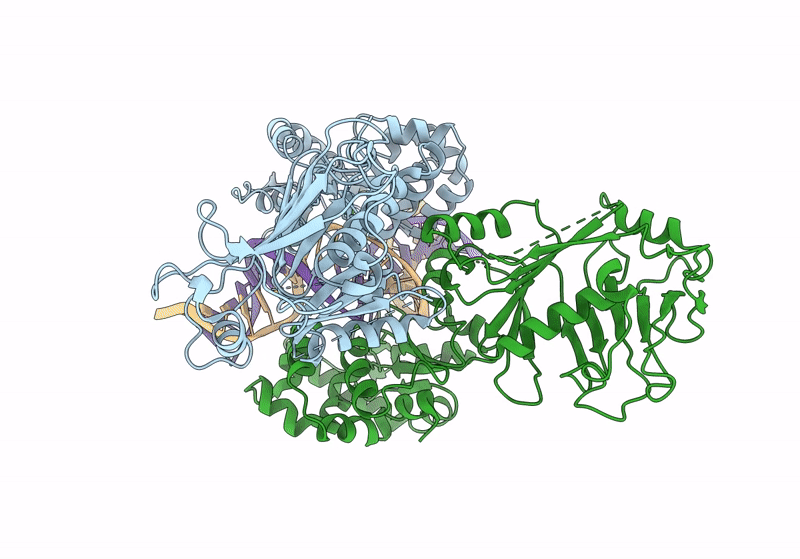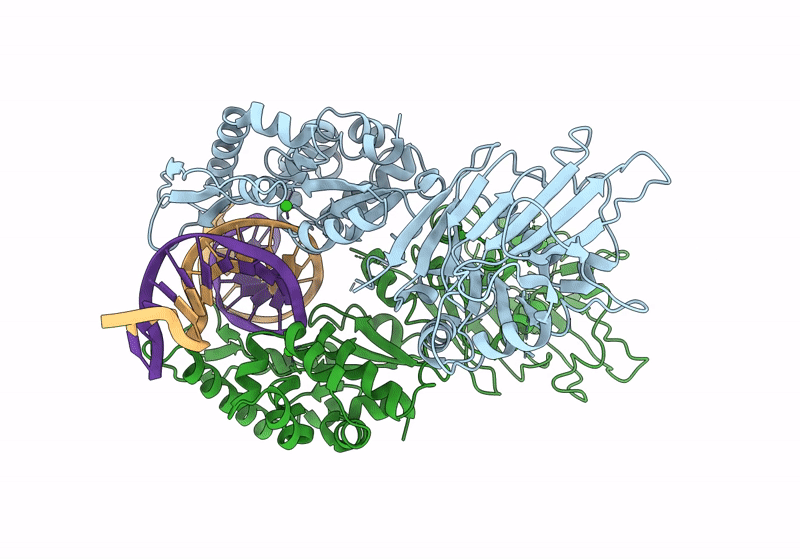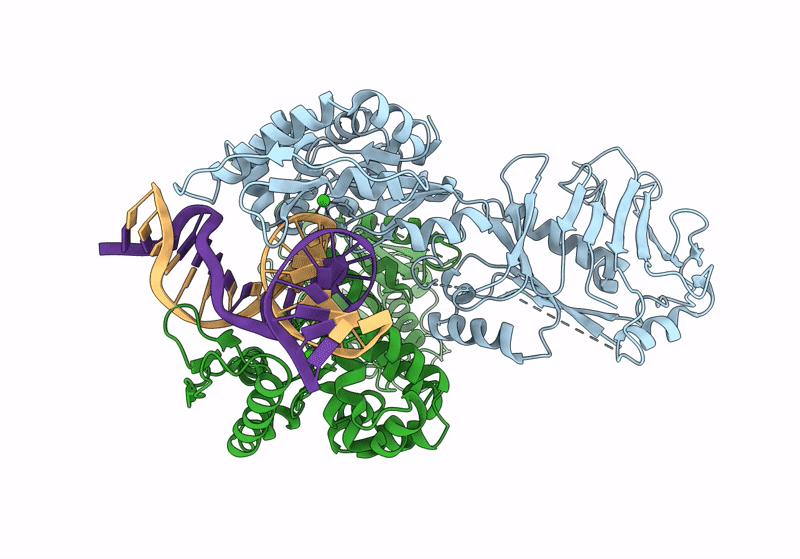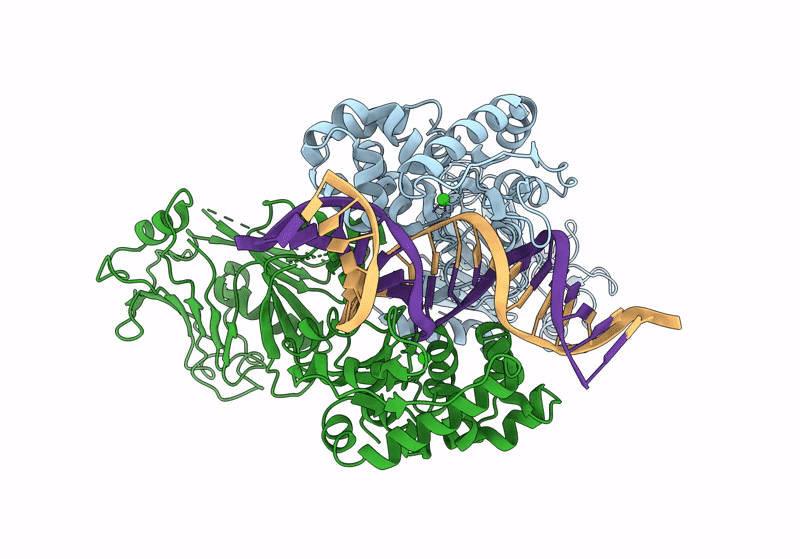
Structure of DNA-bound GajA dimer (focused refinement)
Biological Source:
Source Organism(s):
Bacillus cereus VD045 (Taxon ID: 1053225)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.92 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


