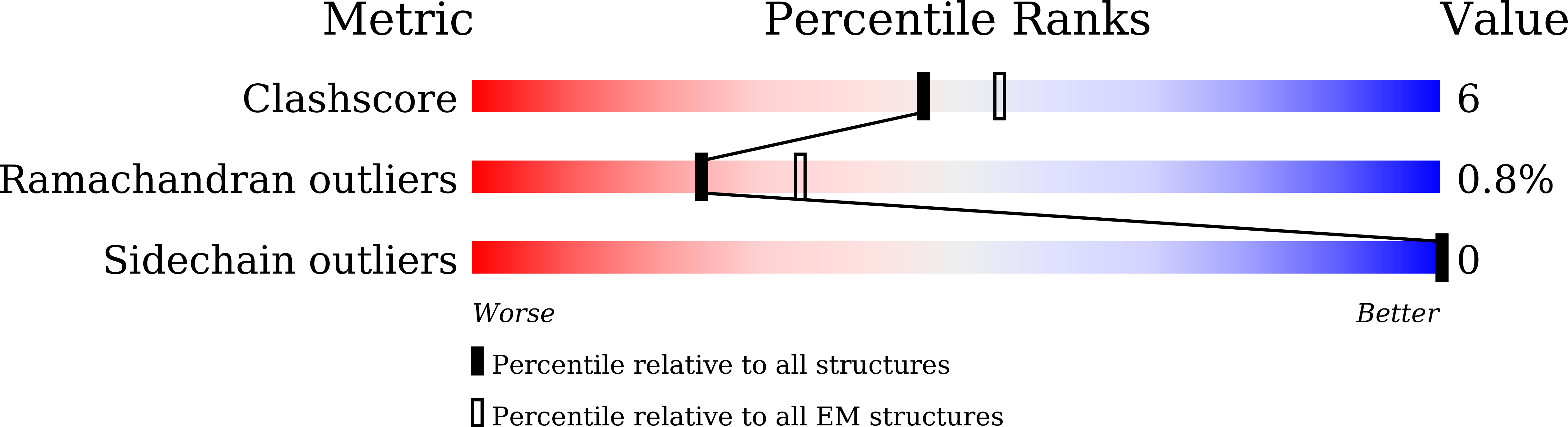
Deposition Date
2023-08-24
Release Date
2023-12-20
Last Version Date
2025-07-02
Entry Detail
PDB ID:
8W4P
Keywords:
Title:
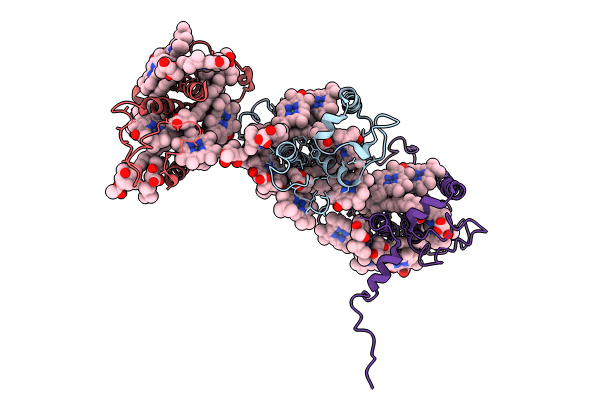
Structure of PSII-FCPII-I/J/K complex in the PSII-FCPII supercomplex from Cyclotella meneghiniana
Biological Source:
Source Organism(s):
Stephanocyclus meneghinianus (Taxon ID: 29205)
Method Details:
Experimental Method:
Resolution:
3.48 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE