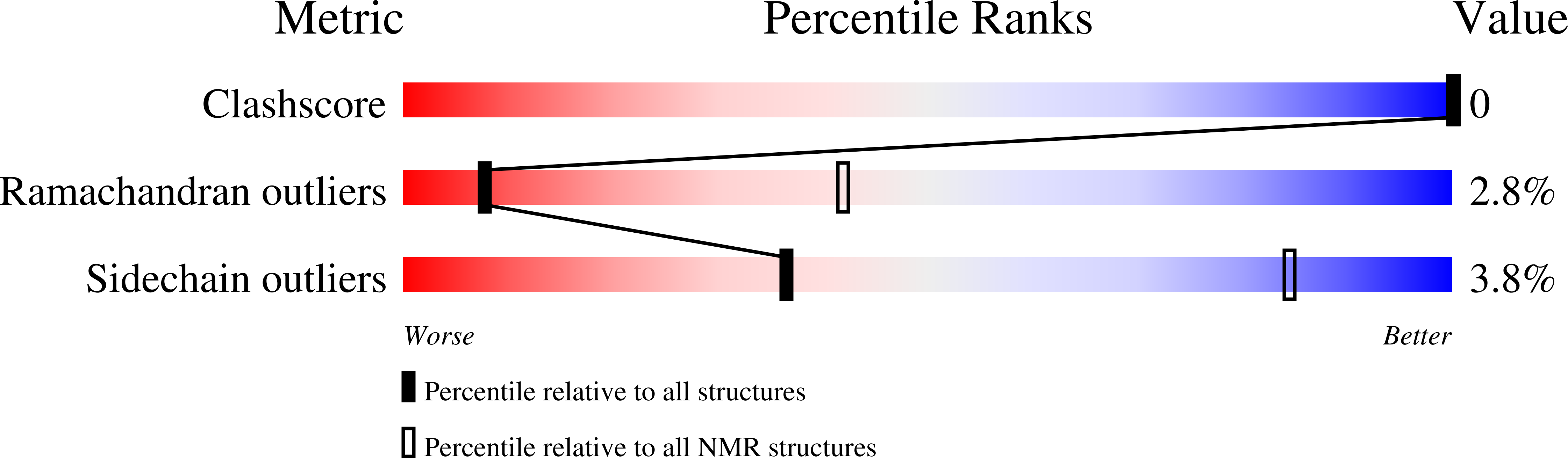
Deposition Date
2024-02-14
Release Date
2024-07-03
Last Version Date
2024-07-31
Method Details:
Experimental Method:
Conformers Calculated:
53799
Conformers Submitted:
20
Selection Criteria:
back calculated data agree with experimental NOESY spectrum