Deposition Date
2024-01-16
Release Date
2024-09-25
Last Version Date
2024-10-02
Entry Detail
PDB ID:
8VPG
Keywords:
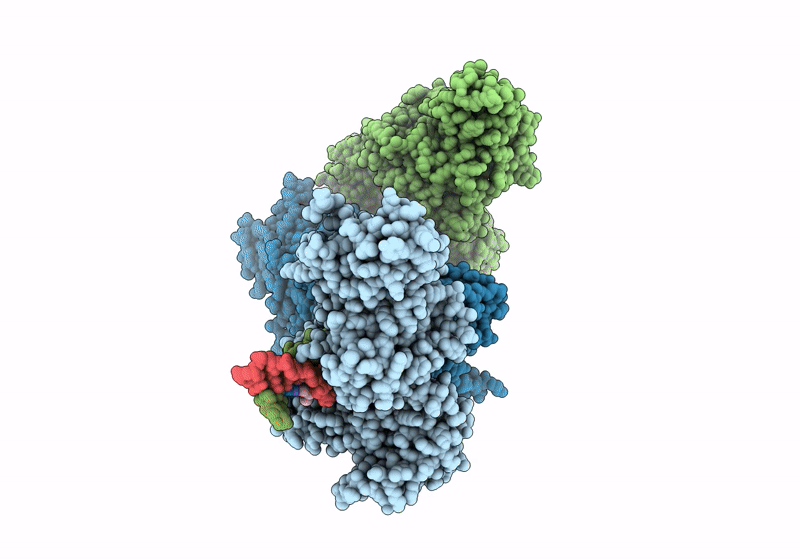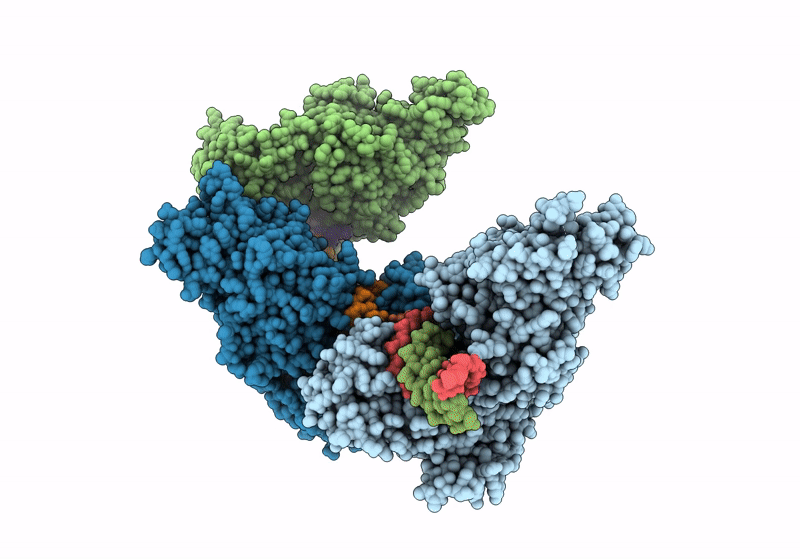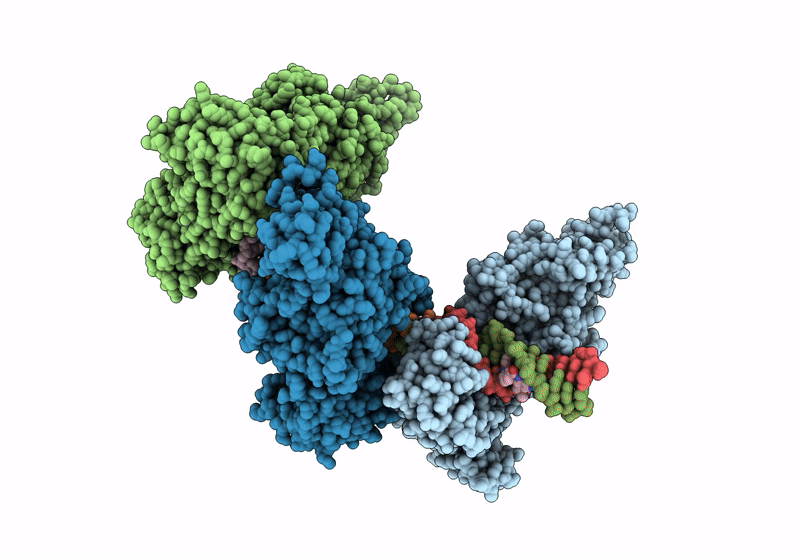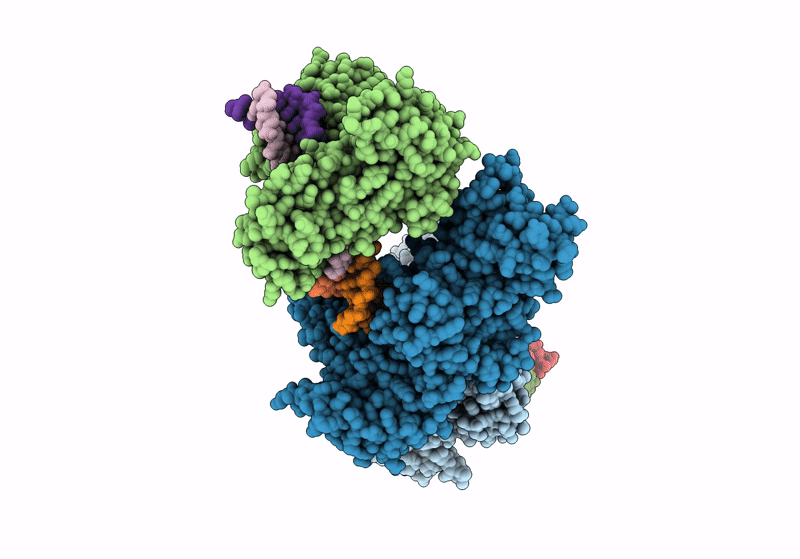
Title:
CamA Adenine Methyltransferase Complexed to Cognate Substrate DNA and Containing Quinoline-based SGI-1027 Analog 455
Biological Source:
Source Organism:
Clostridioides difficile (Taxon ID: 1496)
Host Organism:
Method Details:
Experimental Method:
Resolution:
3.05 Å
R-Value Free:
0.23
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 21 21 21


