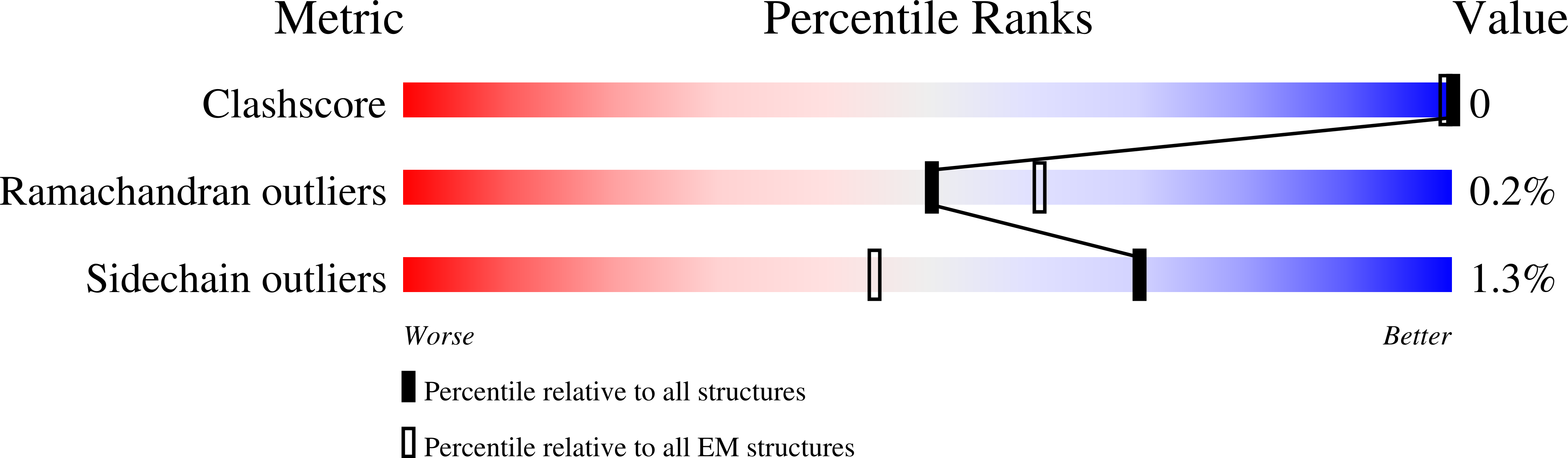
Deposition Date
2023-11-14
Release Date
2024-01-31
Last Version Date
2024-03-20
Entry Detail
PDB ID:
8UZ1
Keywords:
Title:
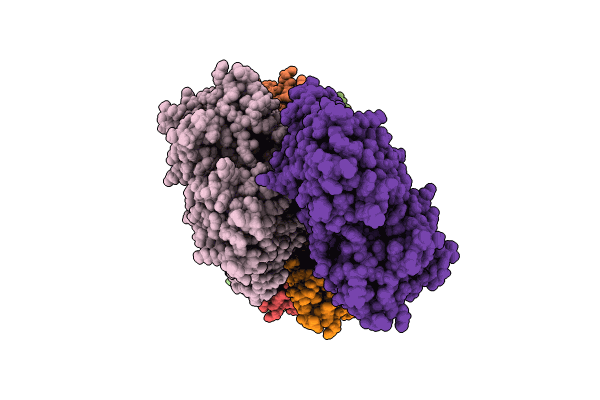
Straight actin filament from Arp2/3 branch junction sample (ADP-BeFx)
Biological Source:
Source Organism(s):
Oryctolagus cuniculus (Taxon ID: 9986)
Method Details:
Experimental Method:
Resolution:
3.60 Å
Aggregation State:
PARTICLE
Reconstruction Method:
HELICAL