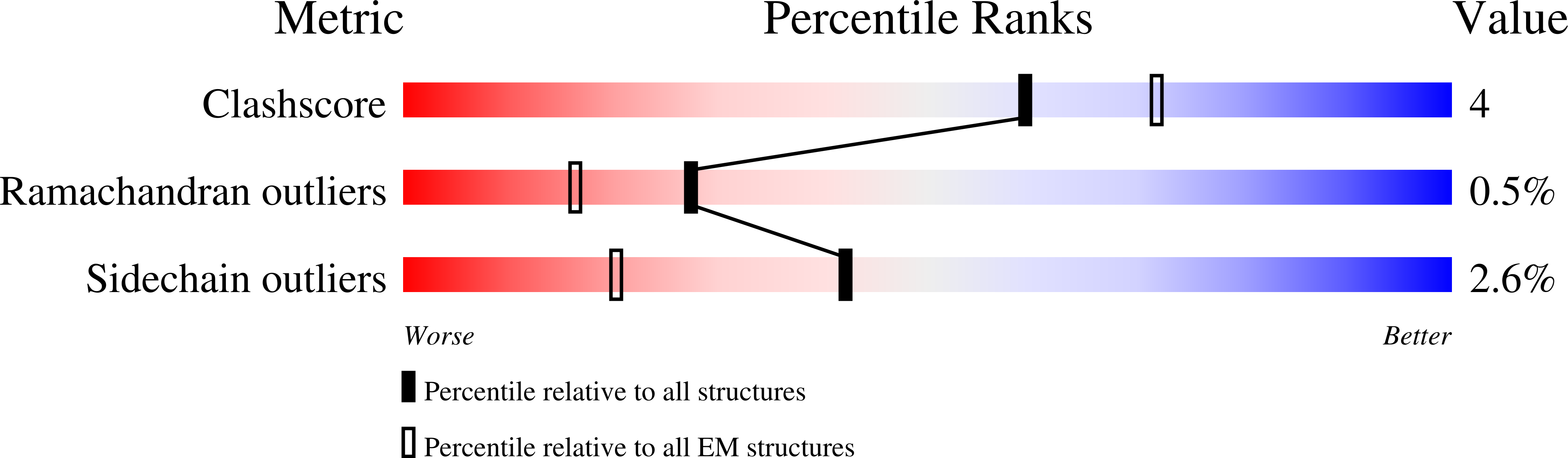
Deposition Date
2023-11-10
Release Date
2024-10-09
Last Version Date
2025-05-14
Entry Detail
PDB ID:
8UXT
Keywords:
Title:
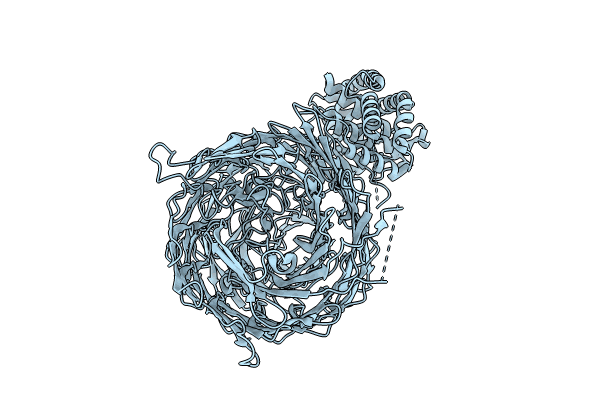
Acinetobacter baumannii Tse15 Rhs effector, toxin cleavage mutant (D1369N, D1391N)
Biological Source:
Source Organism(s):
Acinetobacter baumannii AB307-0294 (Taxon ID: 557600)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
1.77 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE