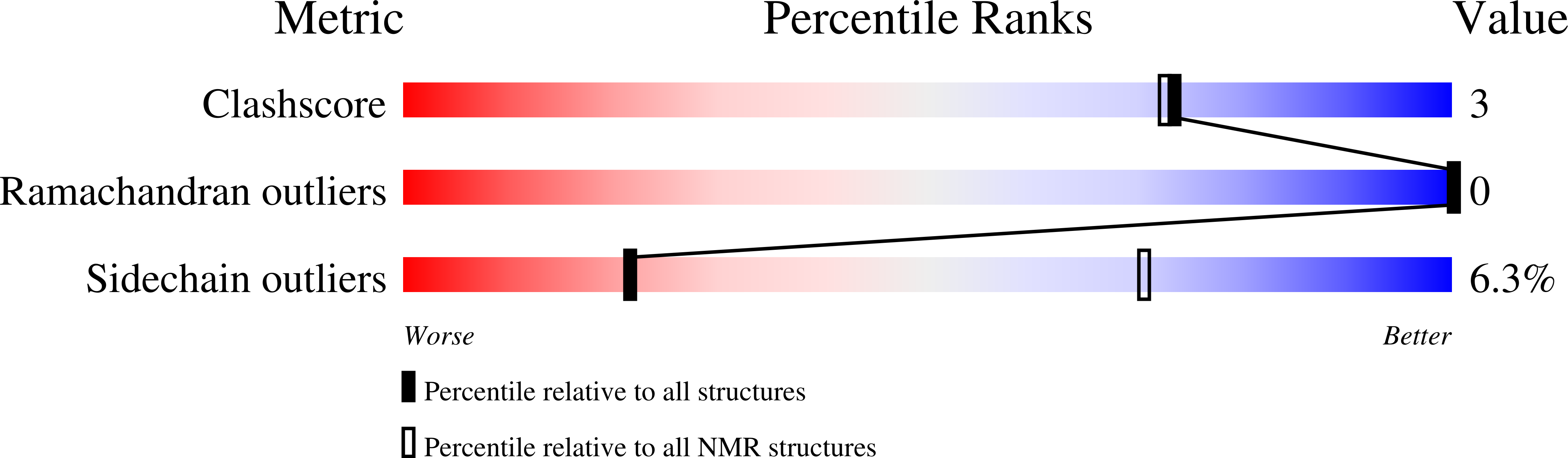
Deposition Date
2023-11-09
Release Date
2024-03-27
Last Version Date
2024-10-30
Method Details:
Experimental Method:
Conformers Calculated:
20
Conformers Submitted:
20
Selection Criteria:
target function