Deposition Date
2023-10-31
Release Date
2024-02-28
Last Version Date
2024-05-08
Entry Detail
PDB ID:
8UUA
Keywords:
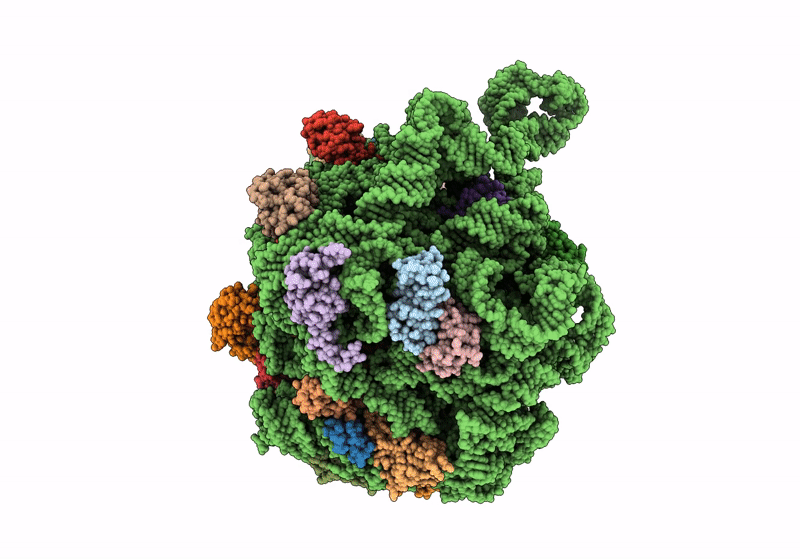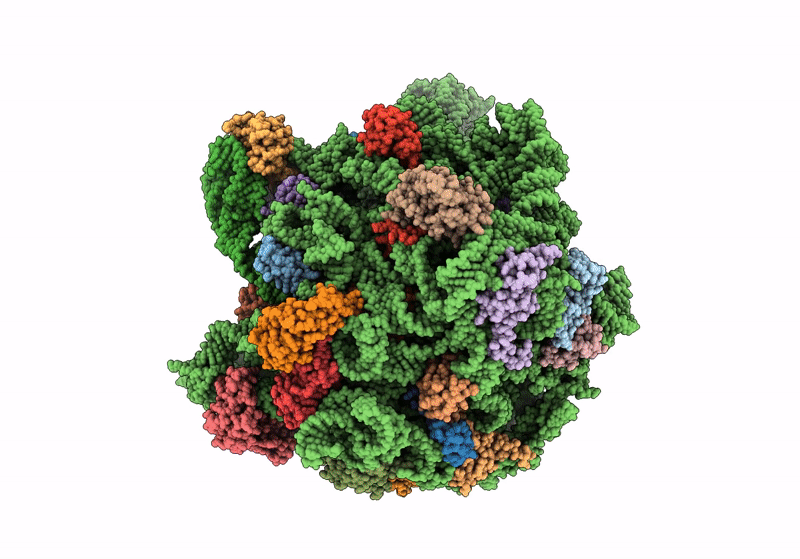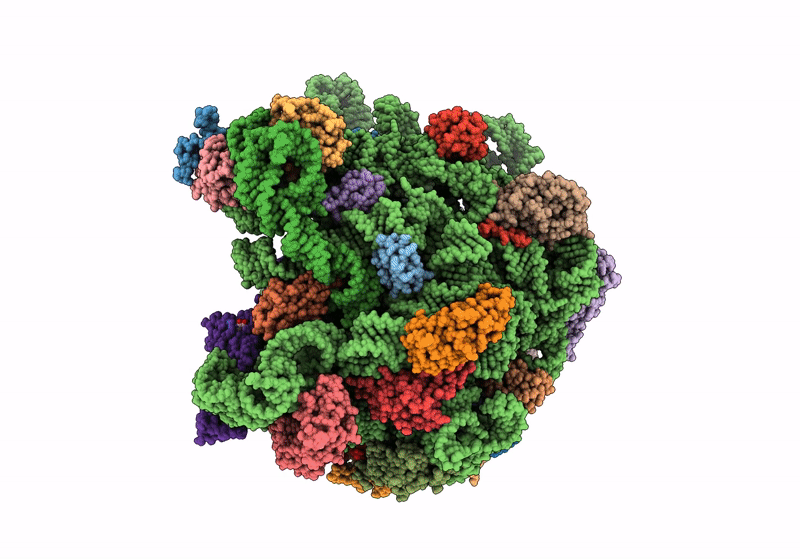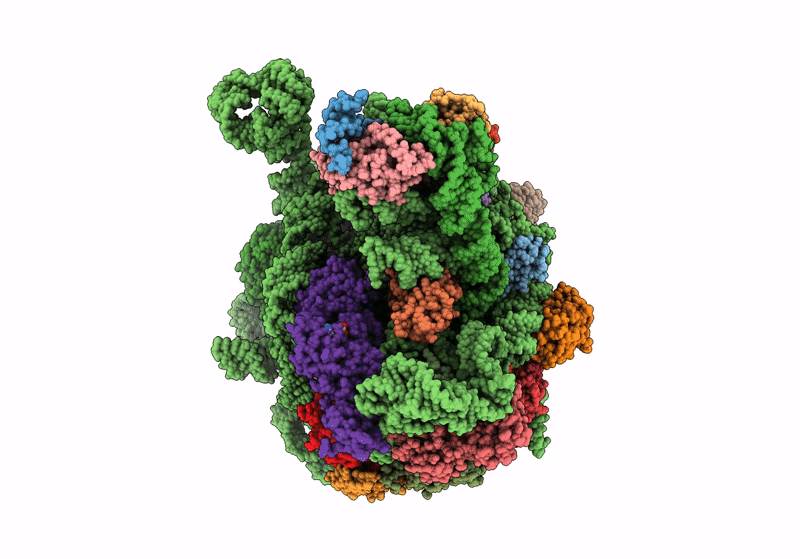
Title:
Cryo-EM structure of the Listeria innocua 50S ribosomal subunit in complex with HflXr (structure III)
Biological Source:
Source Organism(s):
Listeria monocytogenes serovar 1/2a (strain ATCC BAA-679 / EGD-e) (Taxon ID: 169963)
Listeria innocua (Taxon ID: 1642)
Listeria innocua (Taxon ID: 1642)
Method Details:
Experimental Method:
Resolution:
2.70 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


