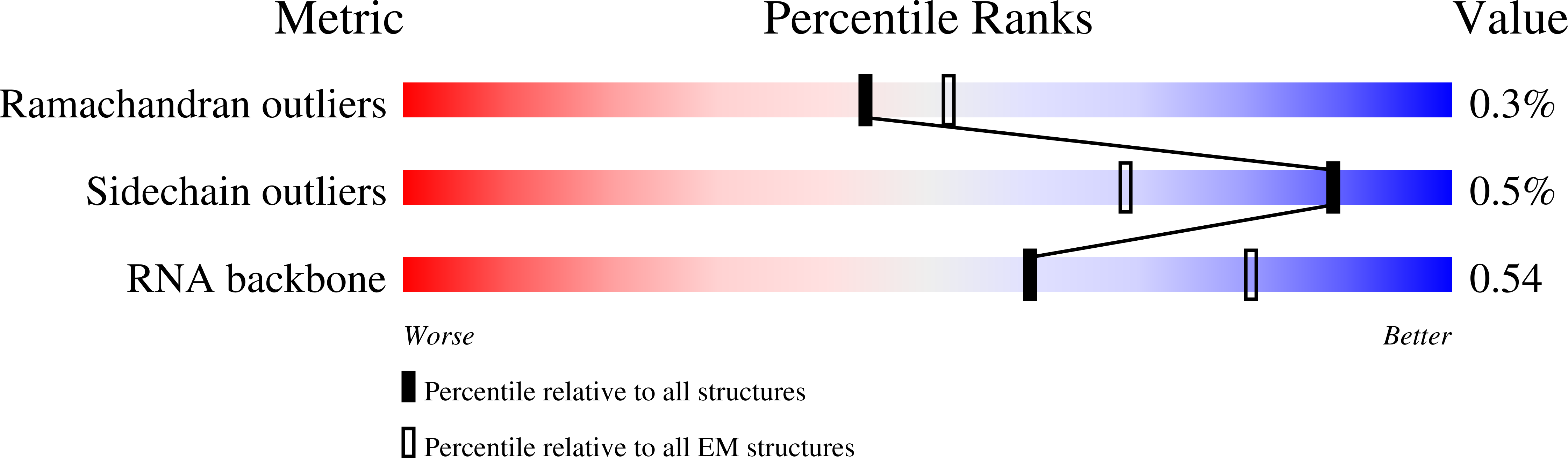Deposition Date
2023-10-23
Release Date
2024-05-29
Last Version Date
2024-12-25
Entry Detail
PDB ID:
8UPO
Keywords:
Title:
Escherichia coli transcription-translation coupled complex class A (TTC-A) containing RfaH bound to ops signal, mRNA with a 21 nt long spacer, and fMet-tRNAs in E-site and P-site of the ribosome
Biological Source:
Source Organism(s):
Escherichia coli (Taxon ID: 562)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
5.50 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE