Deposition Date
2023-10-10
Release Date
2024-10-23
Last Version Date
2025-06-25
Entry Detail
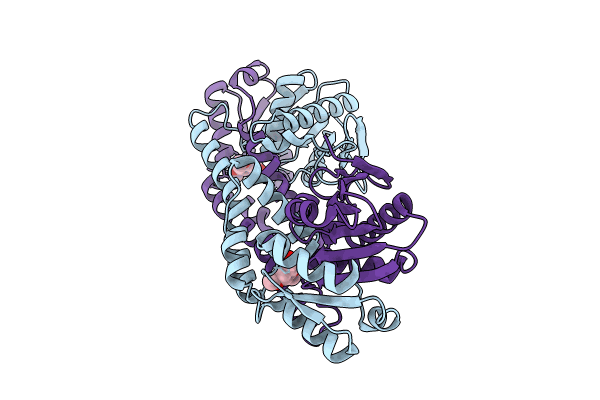
PDB ID:
8UIX
Keywords:
Title:
FphE, Staphylococcus aureus fluorophosphonate-binding serine hydrolases E, boronic acid-based compound Y43 bound
Biological Source:
Source Organism(s):
Staphylococcus aureus subsp. aureus USA300 (Taxon ID: 1385527)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.39 Å
R-Value Free:
0.27
R-Value Work:
0.19
R-Value Observed:
0.20
Space Group:
C 1 2 1


