Deposition Date
2023-10-06
Release Date
2024-12-18
Last Version Date
2025-03-12
Entry Detail
PDB ID:
8UGW
Keywords:
Title:
Computational design of highly signaling active membrane receptors through de novo solvent-mediated allosteric networks
Biological Source:
Source Organism(s):
Tequatrovirus T4 (Taxon ID: 10665)
Homo sapiens (Taxon ID: 9606)
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
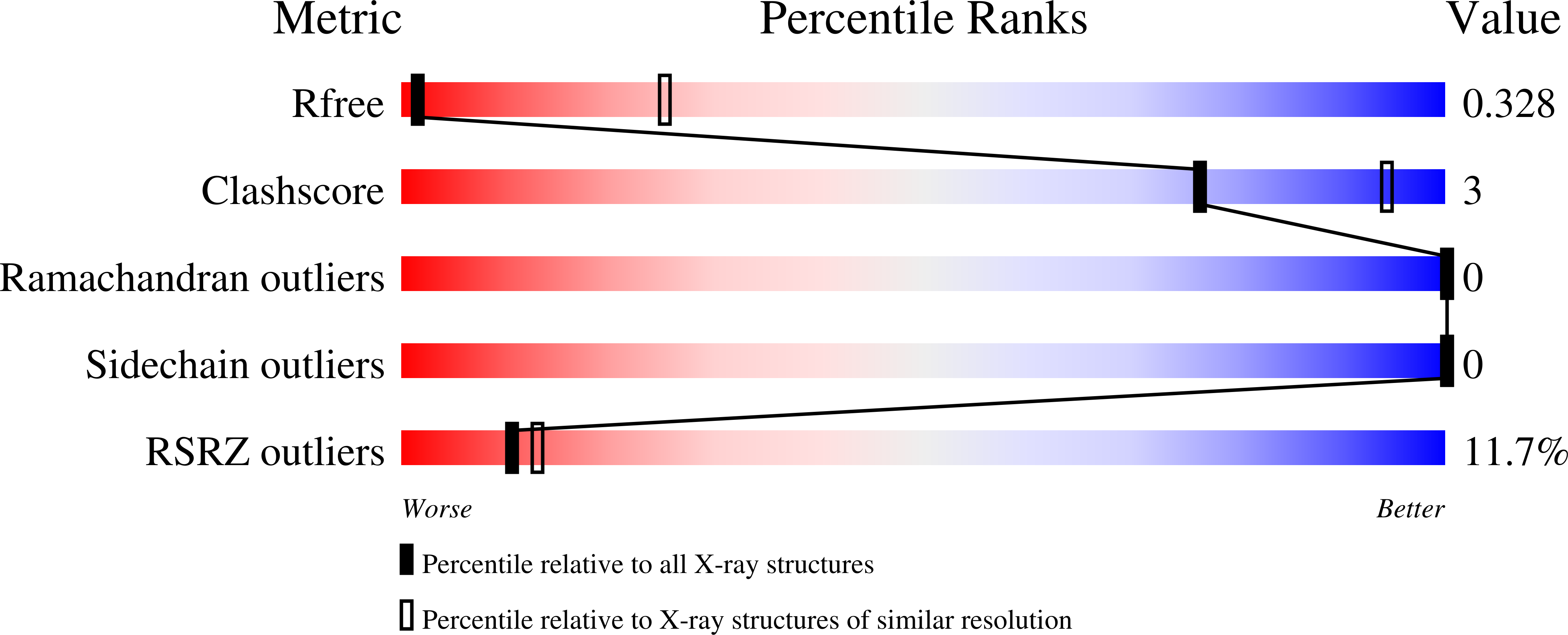
Resolution:
3.90 Å
R-Value Free:
0.33
R-Value Work:
0.30
Space Group:
P 21 21 21