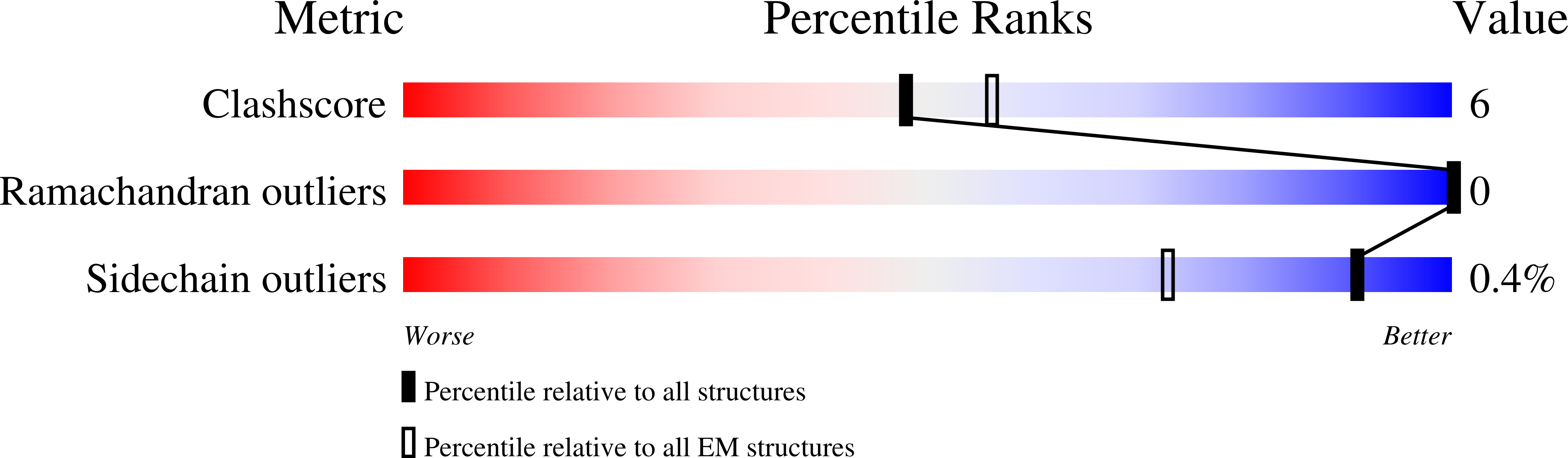
Deposition Date
2023-10-01
Release Date
2023-12-27
Last Version Date
2024-07-10
Entry Detail
PDB ID:
8UEE
Keywords:
Title:
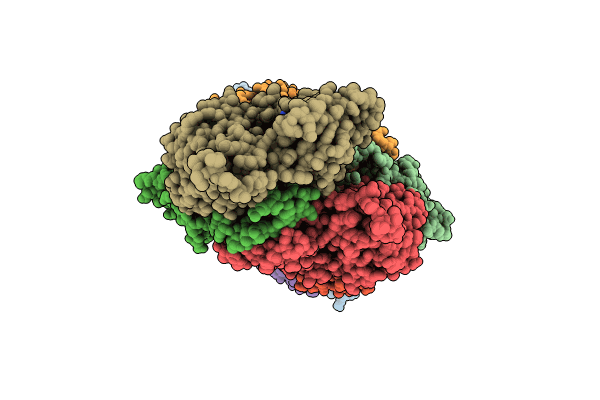
Atomic structure of Salmonella SipA/F-actin complex by cryo-EM
Biological Source:
Source Organism(s):
Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 (Taxon ID: 99287)
Oryctolagus cuniculus (Taxon ID: 9986)
Oryctolagus cuniculus (Taxon ID: 9986)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.20 Å
Aggregation State:
FILAMENT
Reconstruction Method:
HELICAL