Deposition Date
2023-09-19
Release Date
2024-04-24
Last Version Date
2024-07-10
Entry Detail
PDB ID:
8U9O
Keywords:
Title:
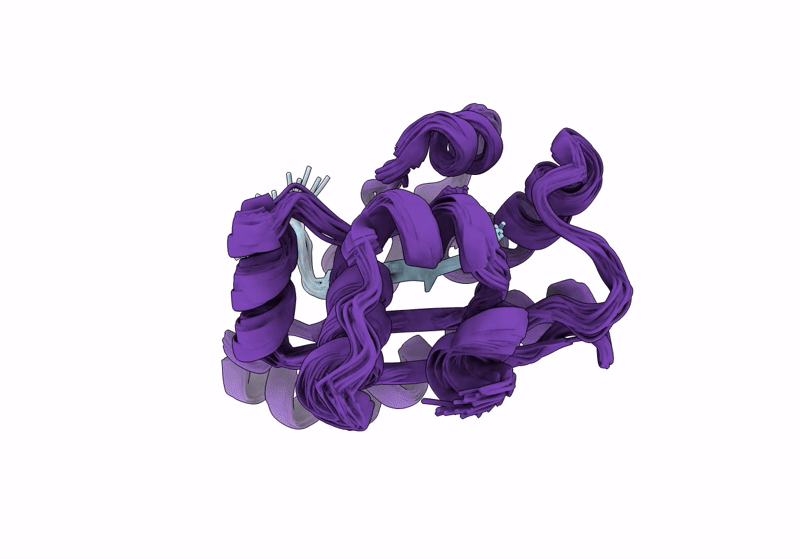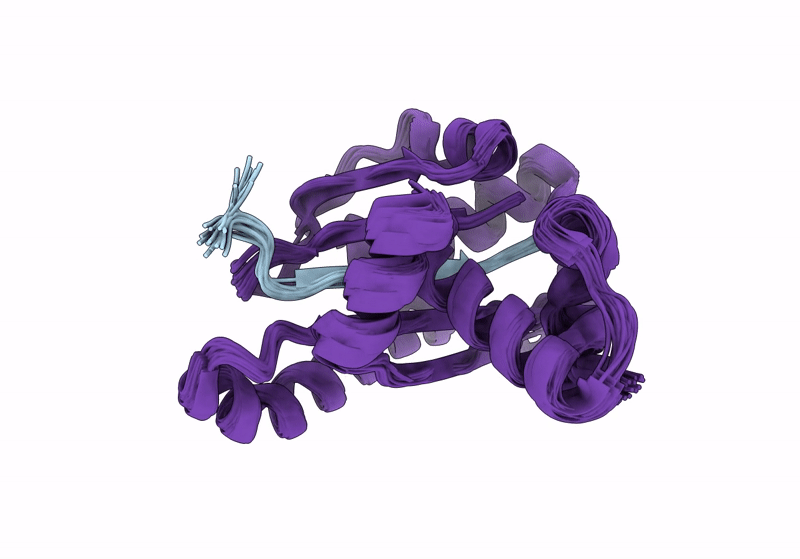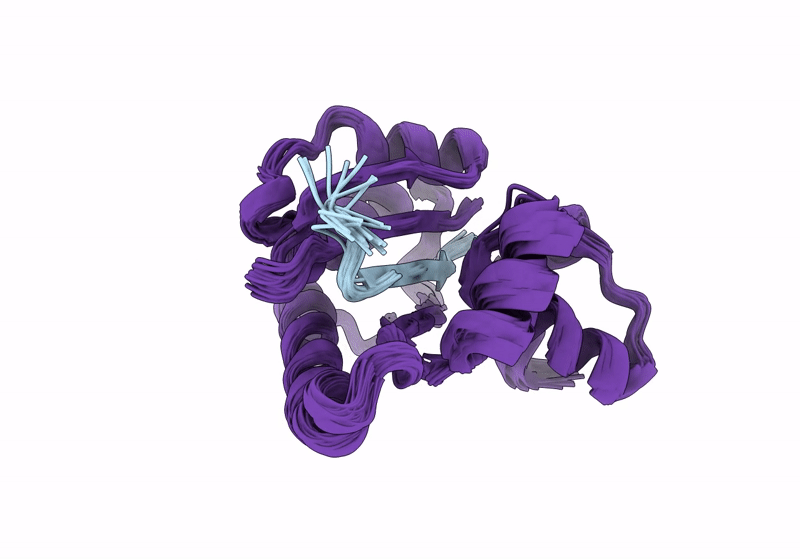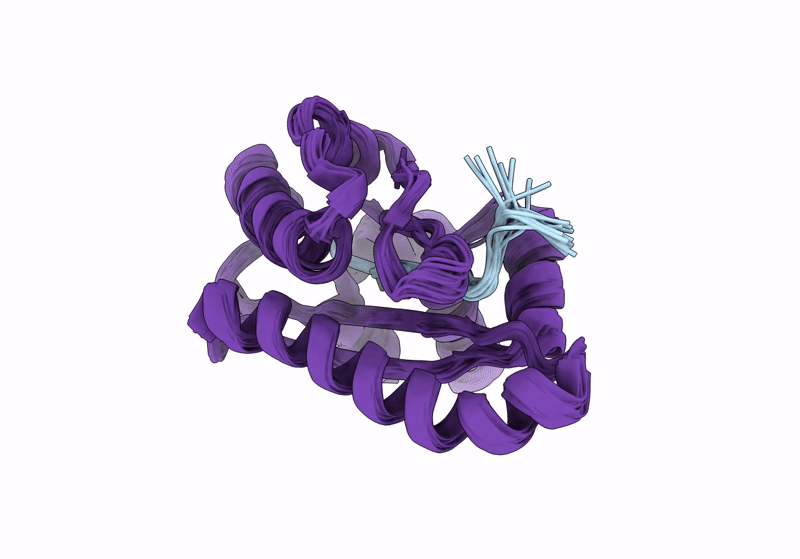
Solution structure of RsgI9 CRE domain from C. thermocellum
Biological Source:
Source Organism:
Acetivibrio thermocellus DSM 1313 (Taxon ID: 637887)
Host Organism:
Method Details:
Experimental Method:
Conformers Calculated:
200
Conformers Submitted:
20
Selection Criteria:
structures with the lowest energy


