Deposition Date
2023-09-18
Release Date
2024-01-31
Last Version Date
2024-02-14
Entry Detail
PDB ID:
8U8O
Keywords:
Title:
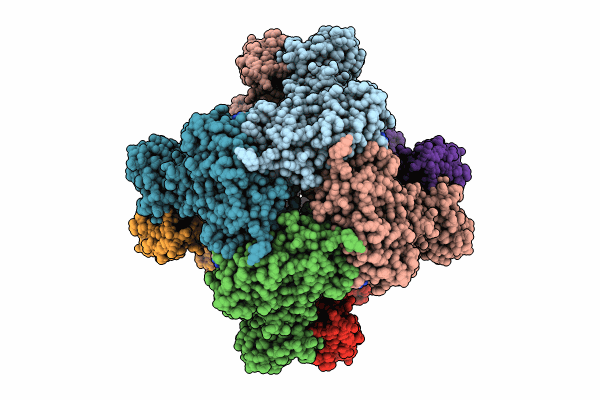
Human retinal variant phosphomimetic IMPDH1(546)-S477D filament bound by ATP, IMP, and NAD+, octamer-centered
Biological Source:
Source Organism:
Homo sapiens (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
Resolution:
2.40 Å
Aggregation State:
FILAMENT
Reconstruction Method:
SINGLE PARTICLE