Deposition Date
2023-09-09
Release Date
2024-05-29
Last Version Date
2024-06-26
Entry Detail
PDB ID:
8U48
Keywords:
Title:
Crystal structure of Bacteroides thetaiotamicron BT1285 D161A-E163A inactive Endoglycosidase in complex with high-mannose N-glycan (Man9GlcNAc2) substrate
Biological Source:
Source Organism:
Bacteroides thetaiotaomicron VPI-5482 (Taxon ID: 226186)
Host Organism:
Method Details:
Experimental Method:
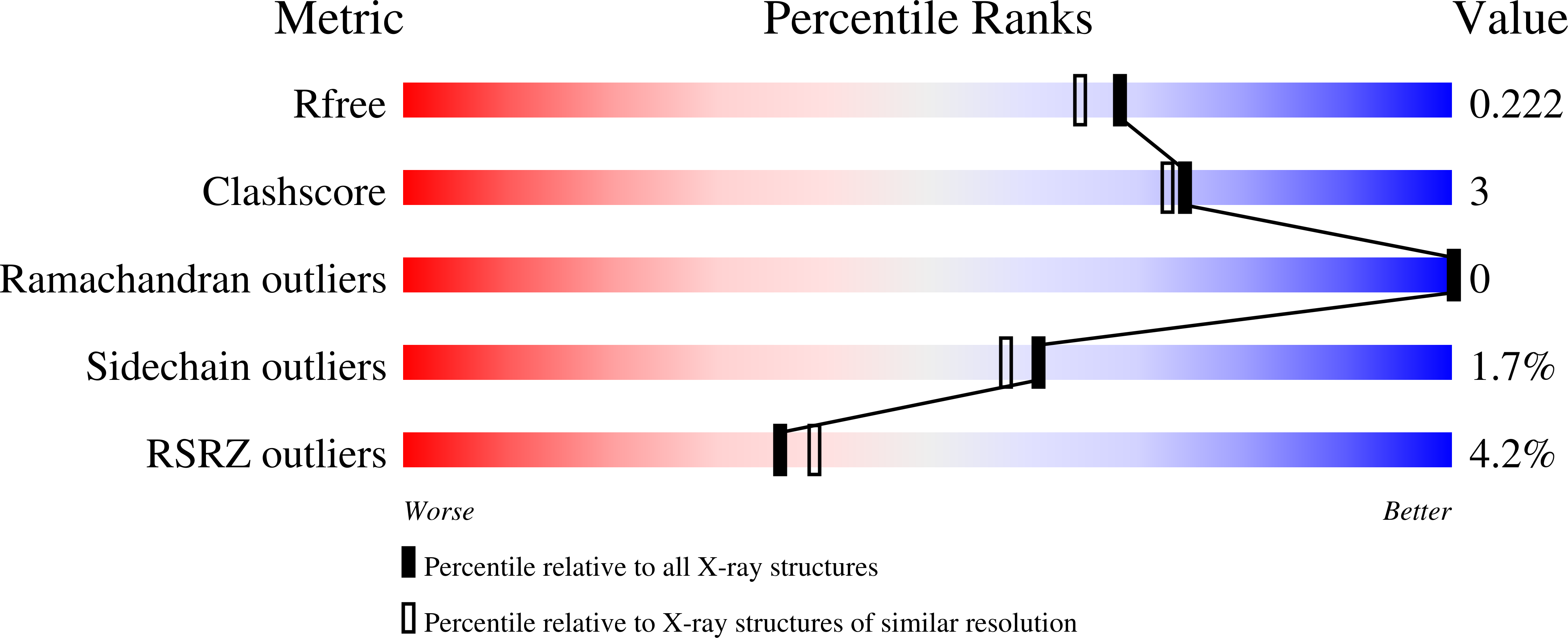
Resolution:
1.90 Å
R-Value Free:
0.22
R-Value Work:
0.18
R-Value Observed:
0.19
Space Group:
P 1 21 1