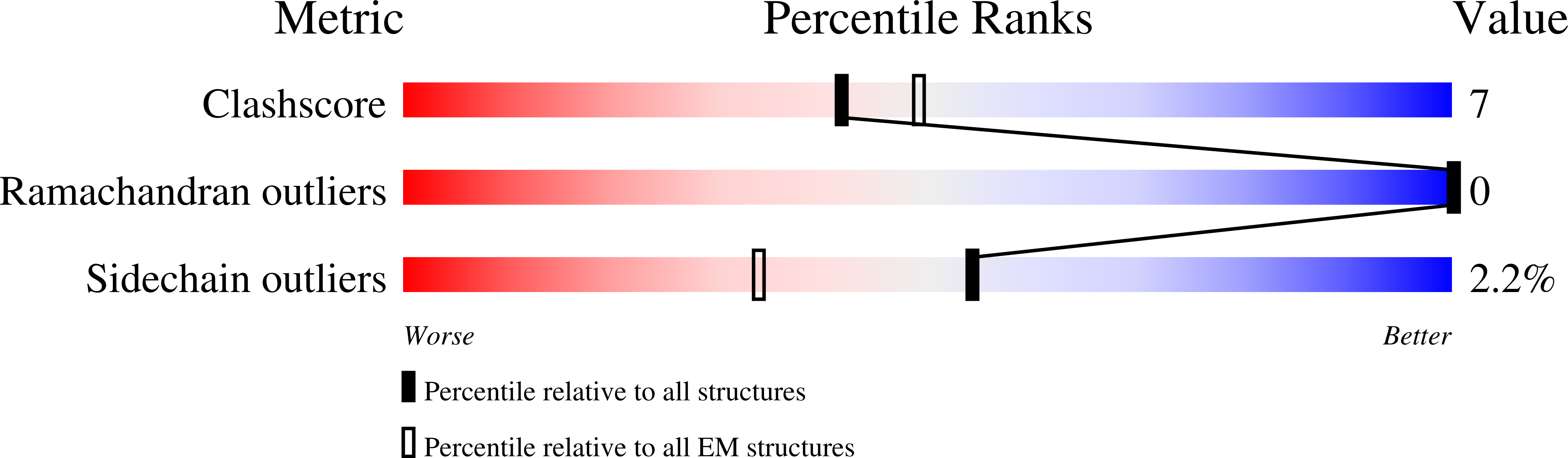
Deposition Date
2023-08-04
Release Date
2024-05-08
Last Version Date
2024-10-30
Entry Detail
PDB ID:
8TPT
Keywords:
Title:
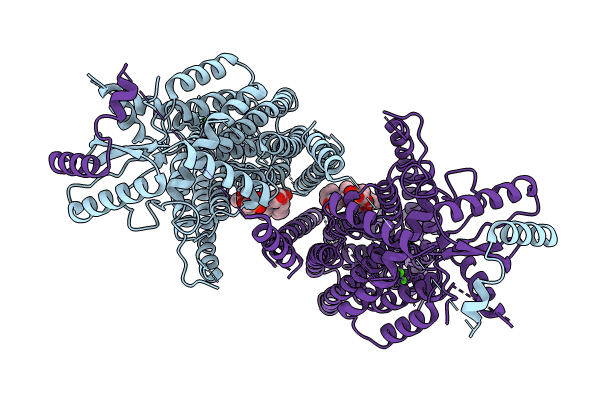
nhTMEM16 A444P mutant in lipid nanodiscs with MSP1E3 scaffold protein in the presence of Ca2+ (long TM6/short TM6)
Biological Source:
Source Organism(s):
Fusarium vanettenii 77-13-4 (Taxon ID: 660122)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.01 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE