Deposition Date
2023-07-05
Release Date
2023-08-09
Last Version Date
2024-10-09
Entry Detail
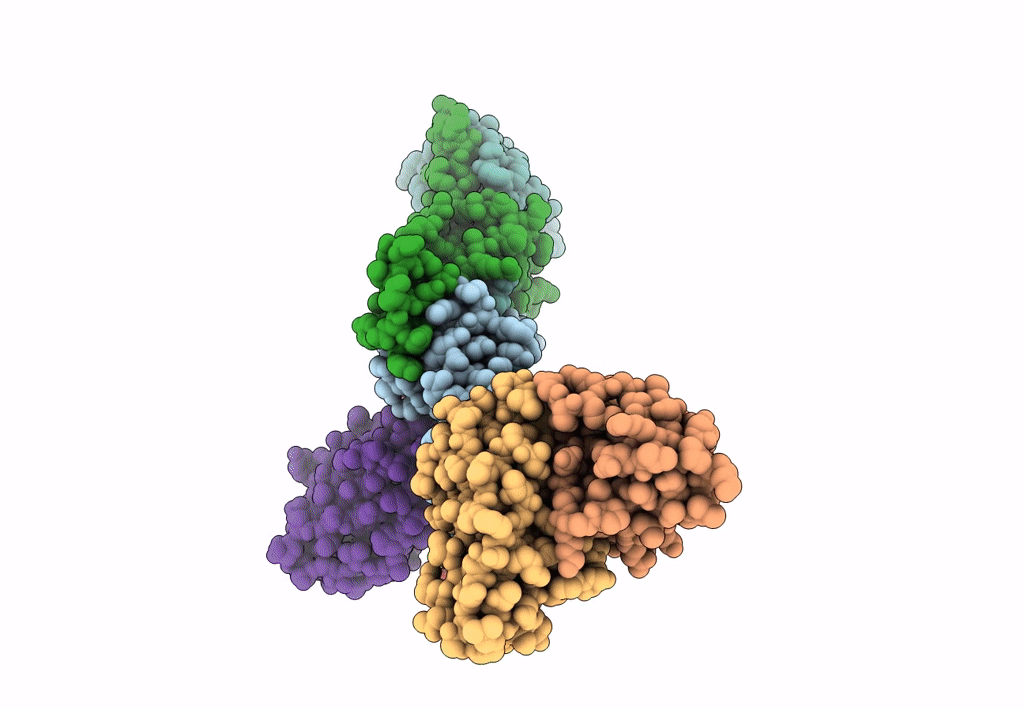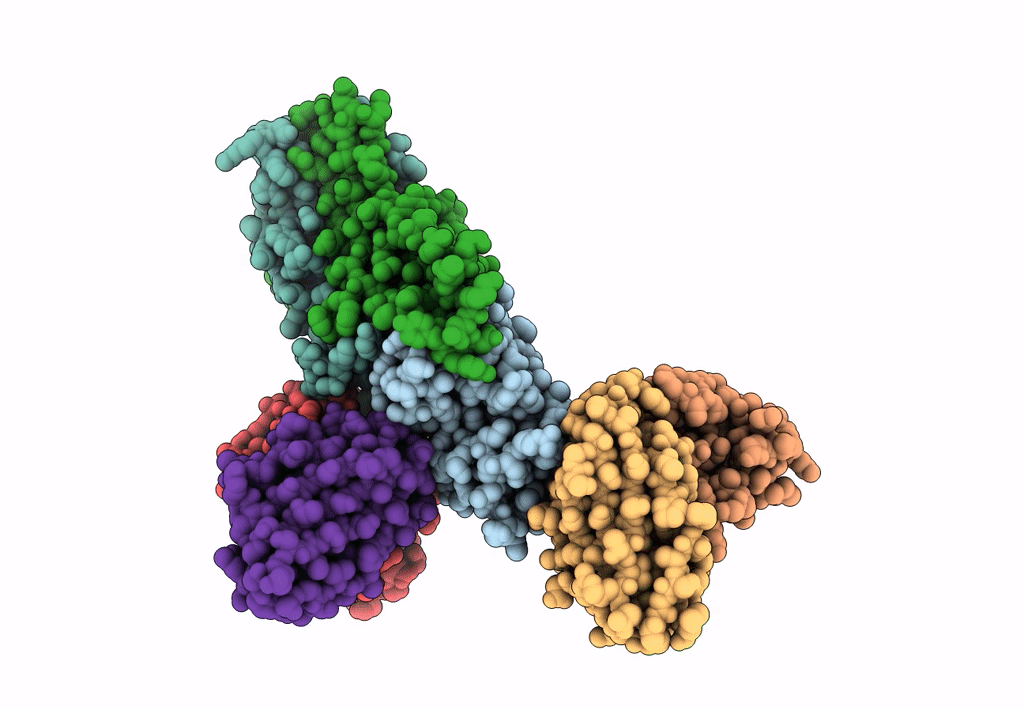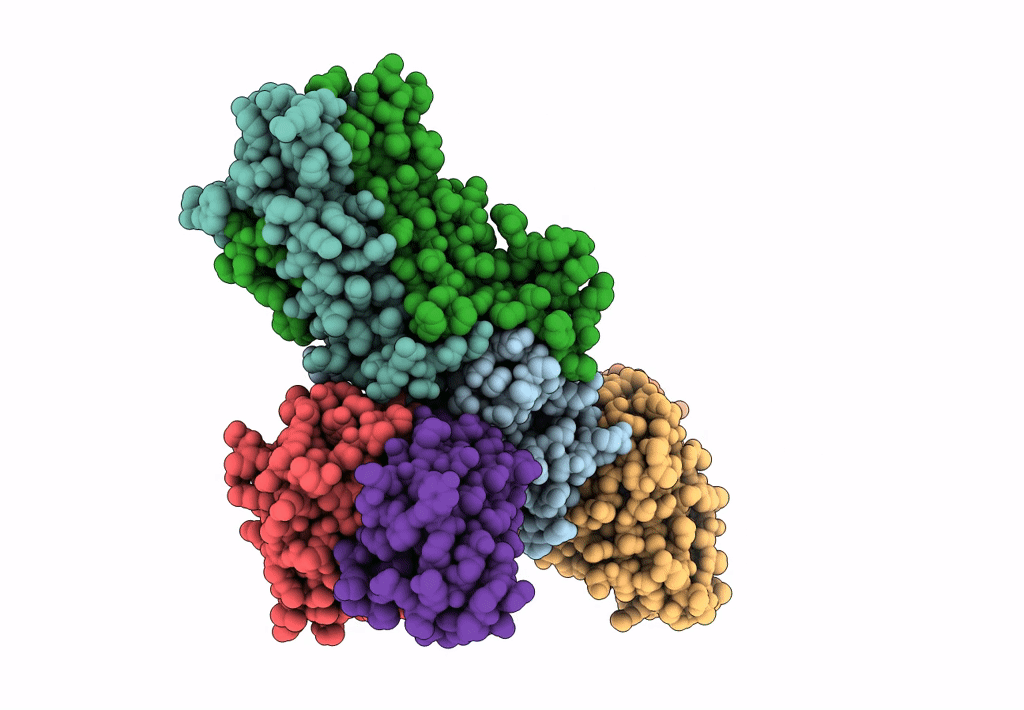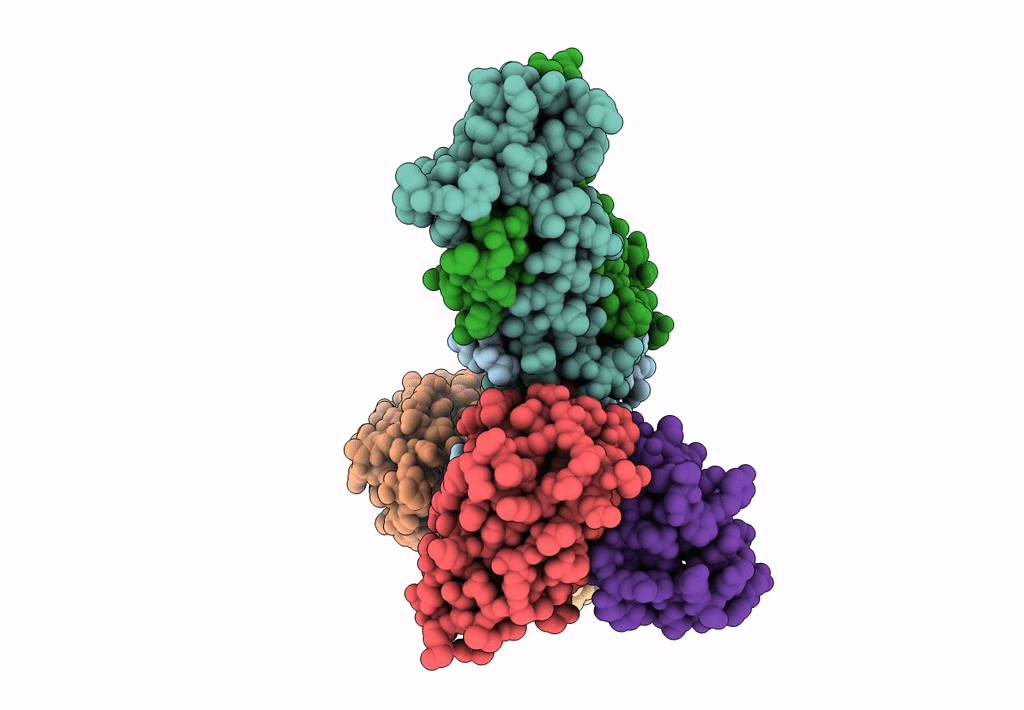
PDB ID:
8TEA
Keywords:
Title:
HCMV Pentamer in complex with CS2pt1p2_A10L Fab and CS3pt1p4_C1L Fab
Biological Source:
Source Organism:
Human betaherpesvirus 5 (Taxon ID: 10359)
Homo sapiens (Taxon ID: 9606)
Homo sapiens (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
Resolution:
3.40 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


