Deposition Date
2023-07-03
Release Date
2024-06-19
Last Version Date
2024-07-17
Entry Detail
PDB ID:
8TDH
Keywords:
Title:
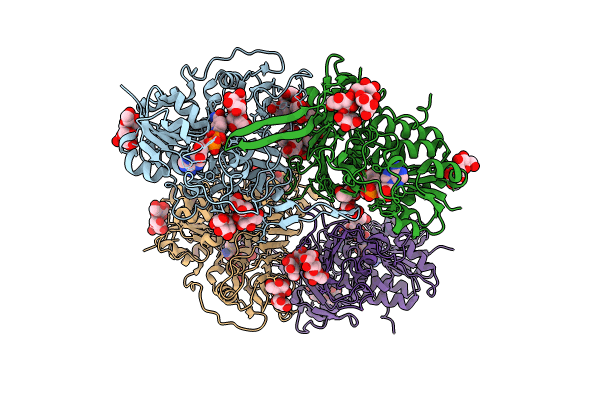
Structure of trehalose bound Alistipes sp. Glucoside-3-dehydrogenase AL3
Biological Source:
Expression System(s):
Method Details:
Experimental Method:
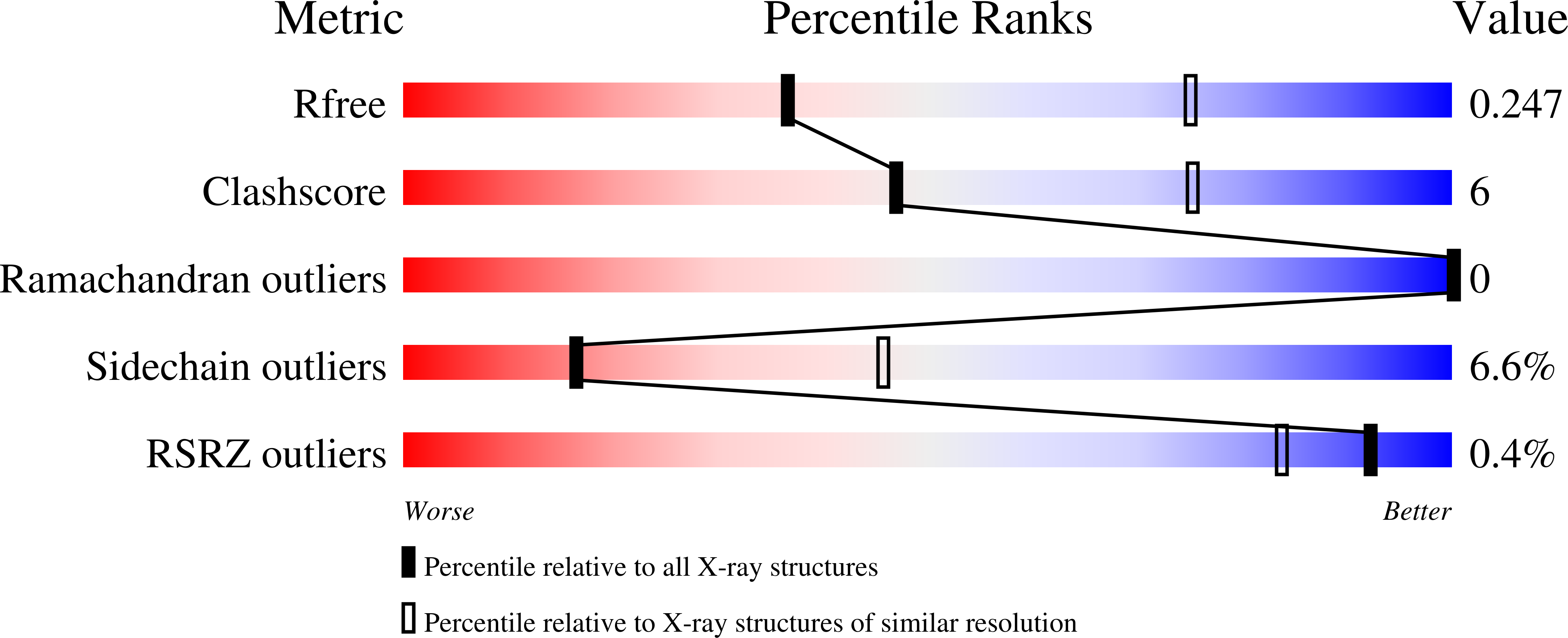
Resolution:
2.95 Å
R-Value Free:
0.24
R-Value Work:
0.19
Space Group:
I 1 2 1