Deposition Date
2023-06-29
Release Date
2024-03-20
Last Version Date
2024-10-16
Entry Detail
PDB ID:
8TBQ
Keywords:
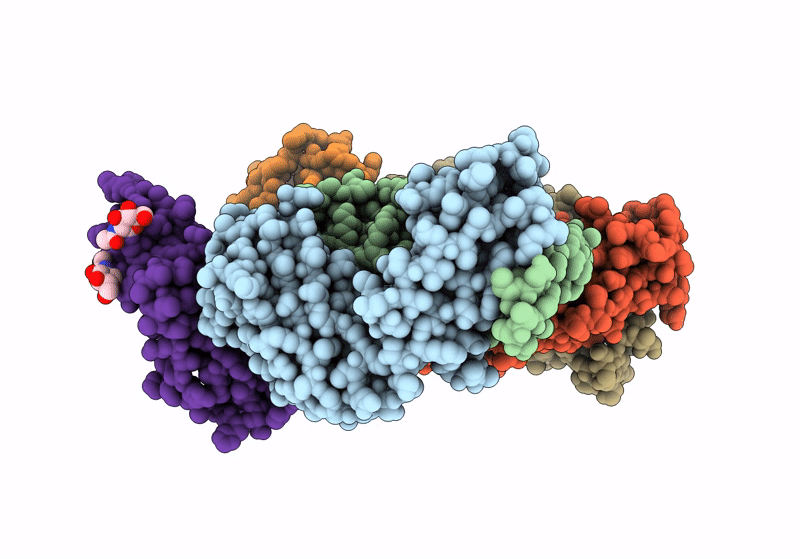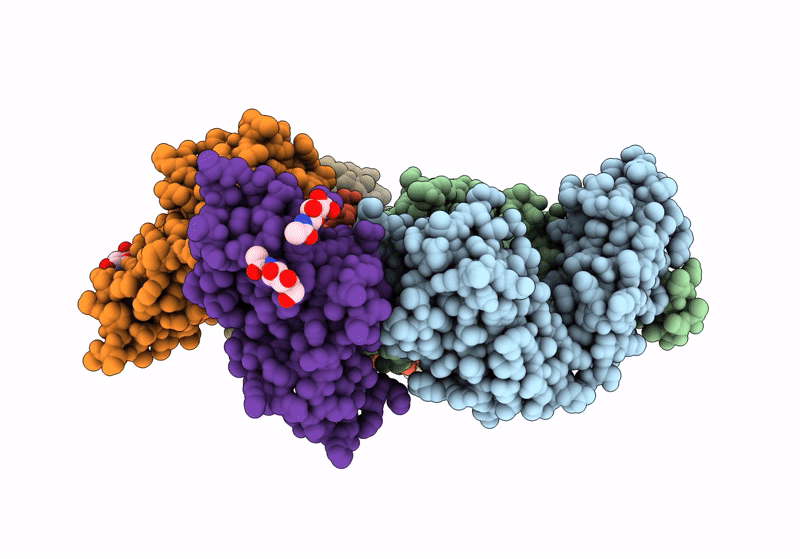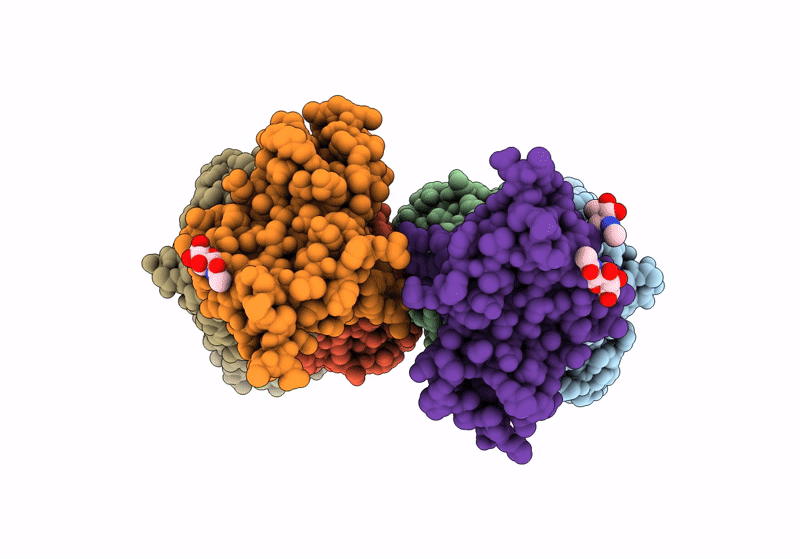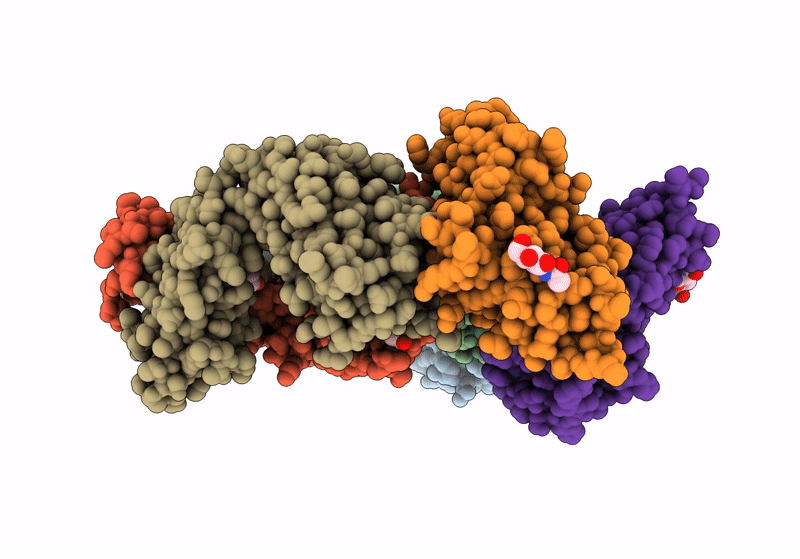
Title:
The crystal structure of human VISTA extra cellular domain in complex with Fab fragment of pH-selective anti-VISTA antibody
Biological Source:
Source Organism:
Homo sapiens (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
Resolution:
2.59 Å
R-Value Free:
0.29
R-Value Work:
0.23
Space Group:
C 1 2 1


