Deposition Date
2023-06-13
Release Date
2024-08-28
Last Version Date
2025-06-04
Entry Detail
PDB ID:
8T5D
Keywords:
Title:
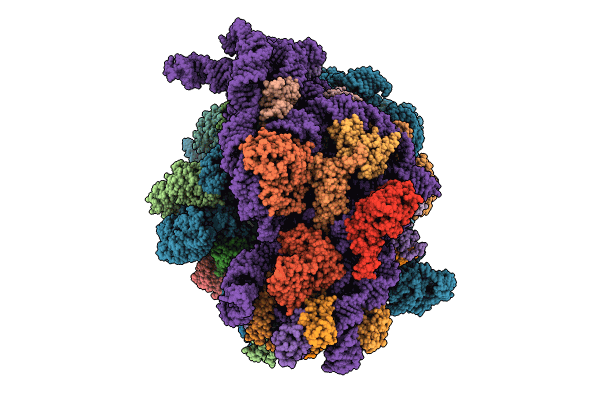
Cryo-EM studies of the interplay between uS2 ribosomal protein and leaderless mRNA during bacterial translation initiation
Biological Source:
Source Organism(s):
Escherichia coli (Taxon ID: 562)
Bacteriophage sp. (Taxon ID: 38018)
Bacteriophage sp. (Taxon ID: 38018)
Expression System(s):
Method Details:
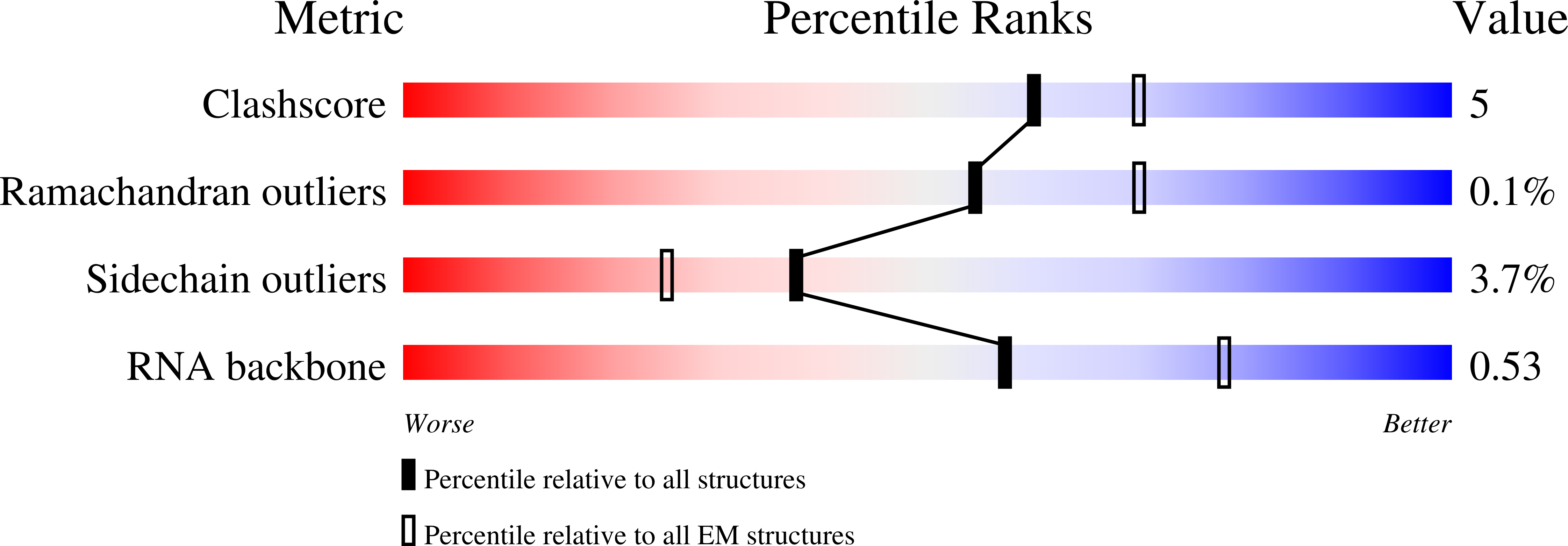
Experimental Method:
Resolution:
3.20 Å
Aggregation State:
CELL
Reconstruction Method:
SINGLE PARTICLE