Deposition Date
2023-06-01
Release Date
2023-08-16
Last Version Date
2023-11-15
Entry Detail
PDB ID:
8T0I
Keywords:
Title:
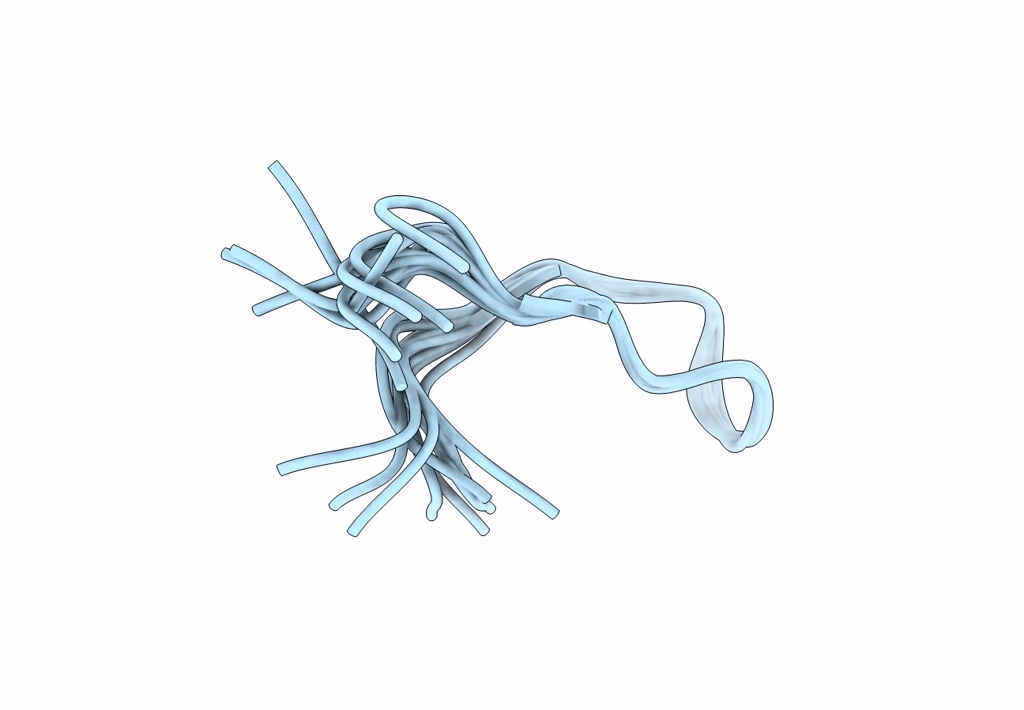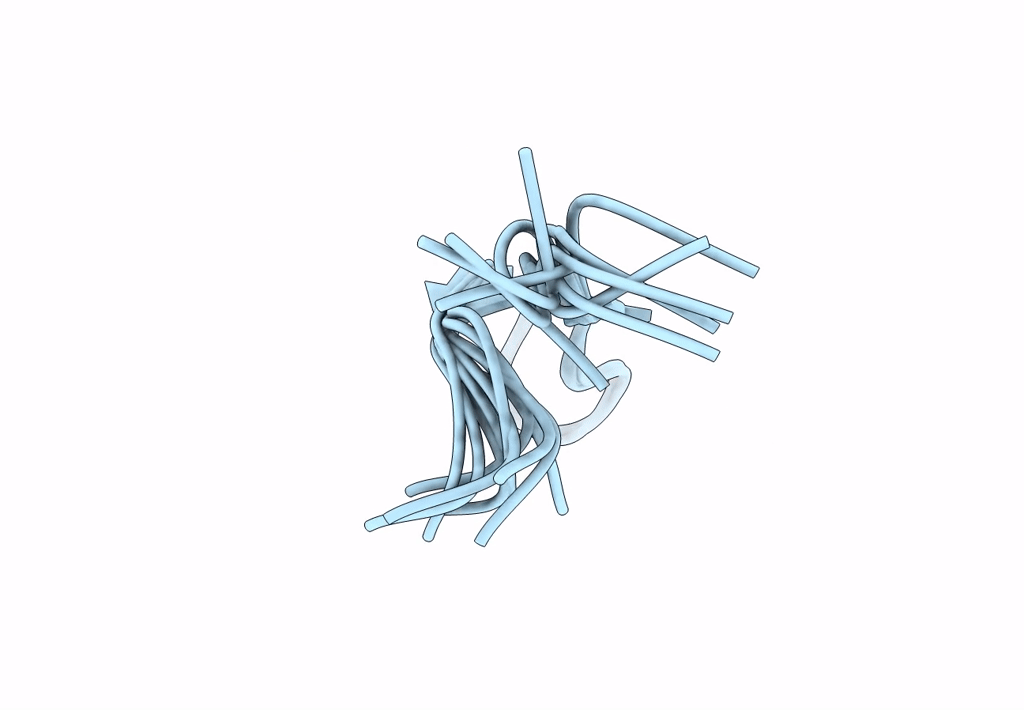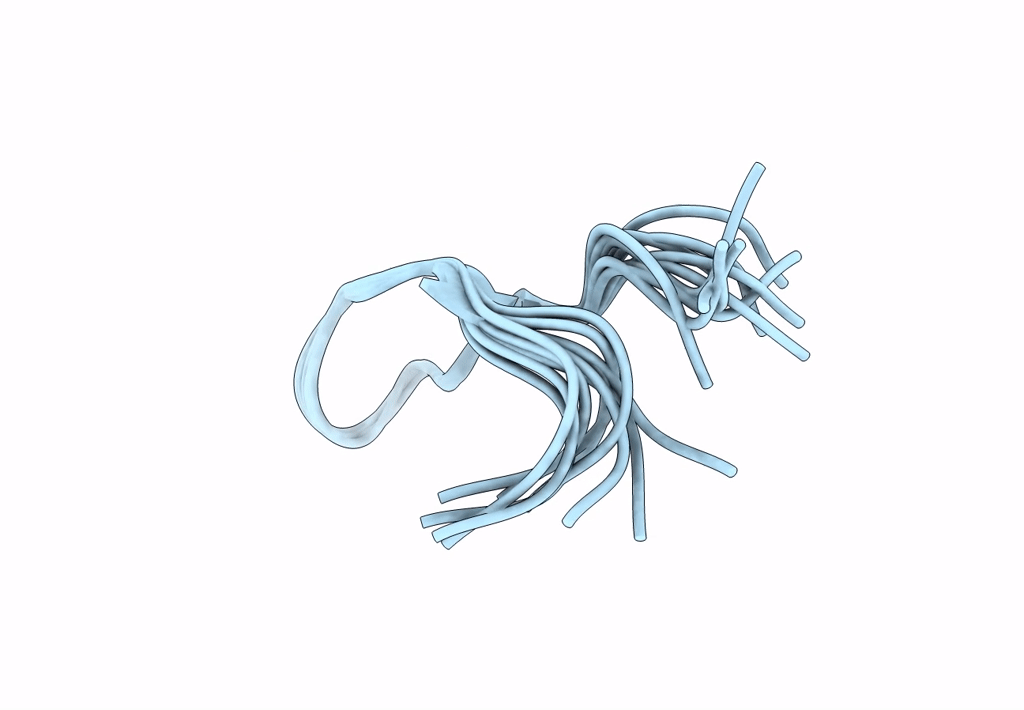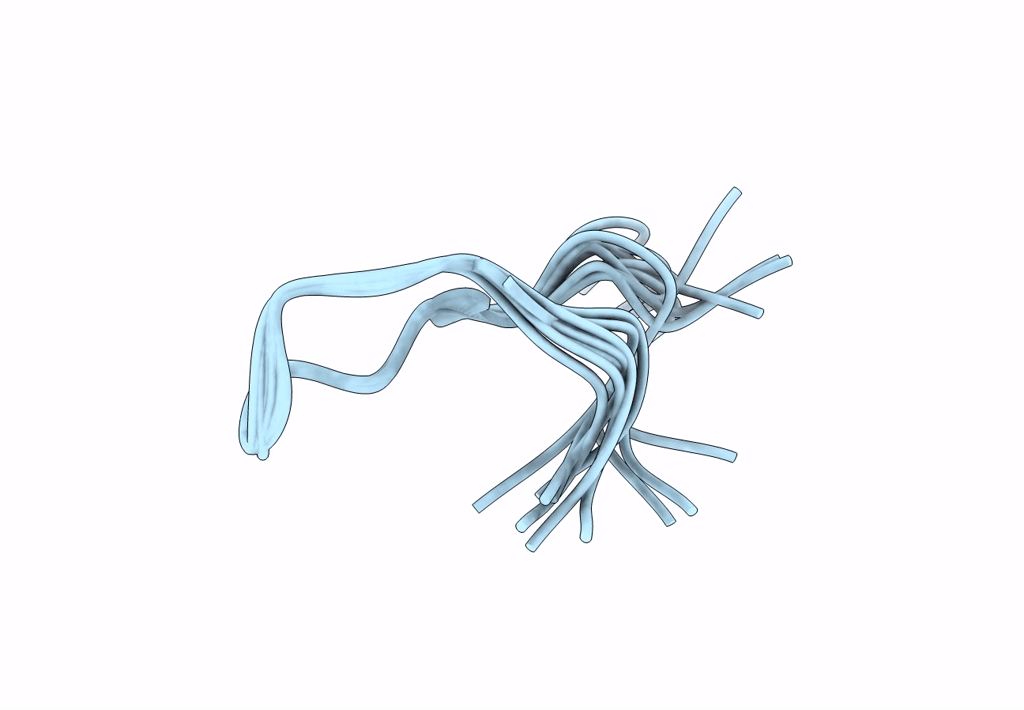
Backbone Dialkylation in Peptide Hairpins: (R)-Ethylpropylglycine variant
Biological Source:
Source Organism(s):
Streptococcus sp. group G (Taxon ID: 1320)
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
10
Selection Criteria:
structures with the lowest energy


