Deposition Date
2023-05-12
Release Date
2023-08-16
Last Version Date
2023-11-15
Entry Detail
PDB ID:
8SUI
Keywords:
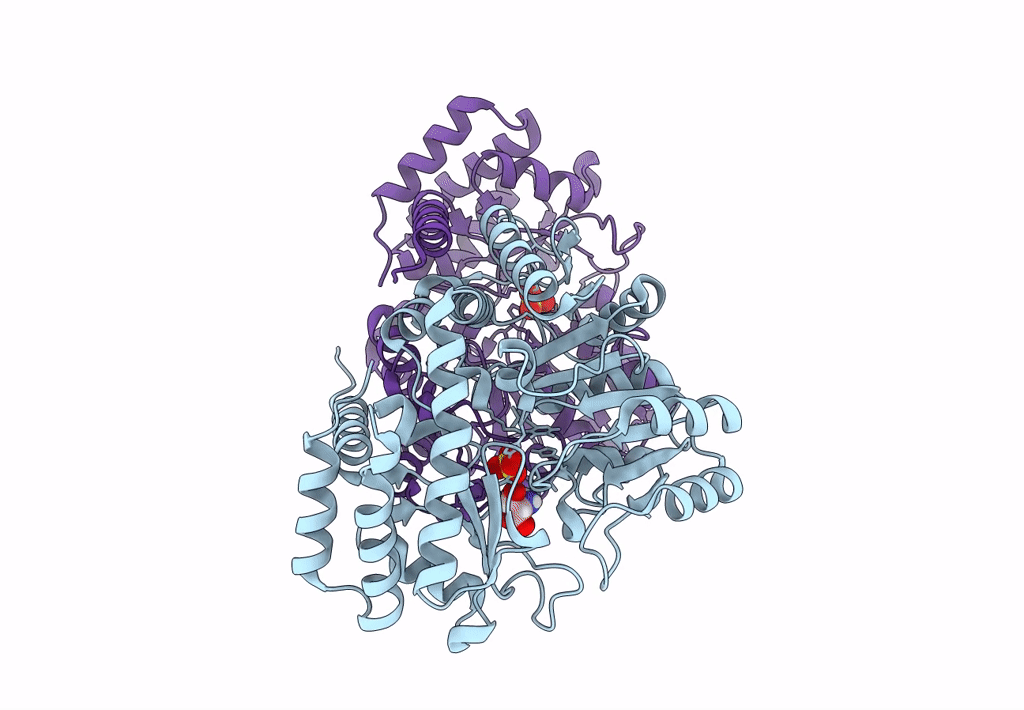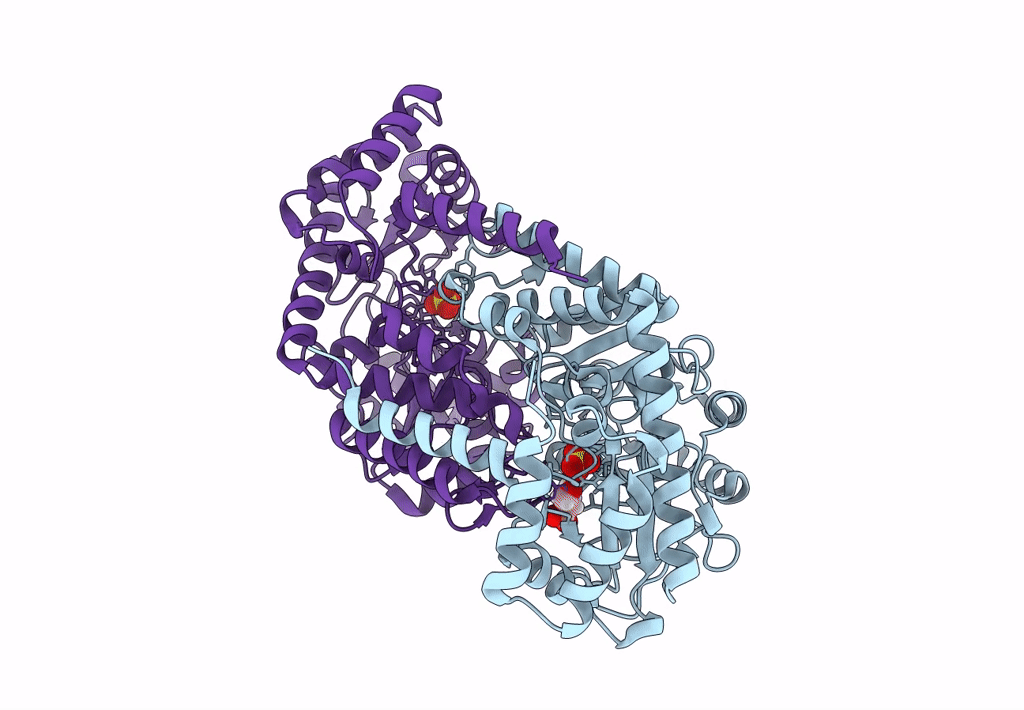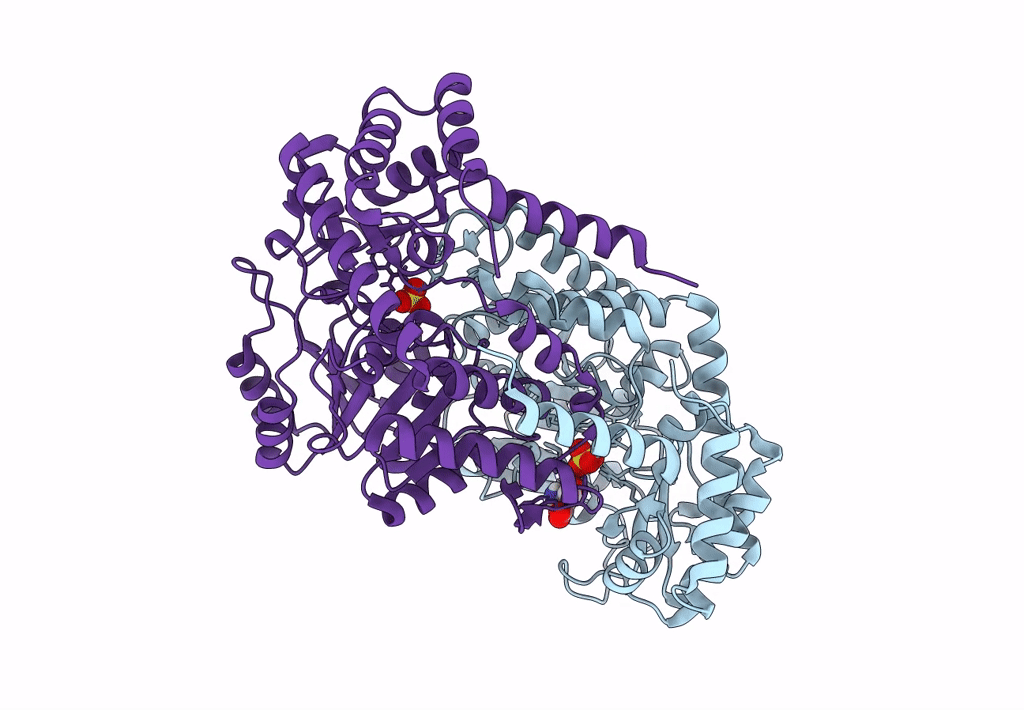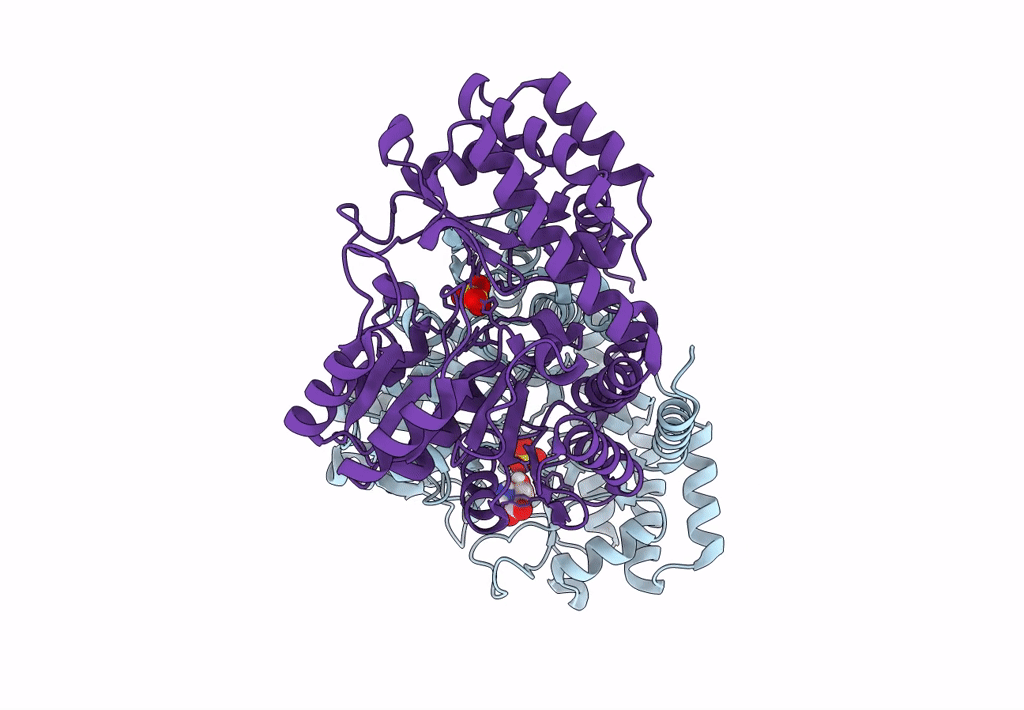
Title:
Joint X-ray/neutron structure of Thermus thermophilus serine hydroxymethyltransferase (TthSHMT) in internal aldimine state with L-Ser bound in a pre-Michalis complex
Biological Source:
Source Organism(s):
Thermus thermophilus HB8 (Taxon ID: 300852)
Expression System(s):
Method Details:
Experimental Method:
R-Value Free:
['0.22
R-Value Work:
['0.20
R-Value Observed:
['?', '?'].00
Space Group:
P 1 21 1


