Deposition Date
2023-04-11
Release Date
2024-07-03
Last Version Date
2025-01-22
Entry Detail
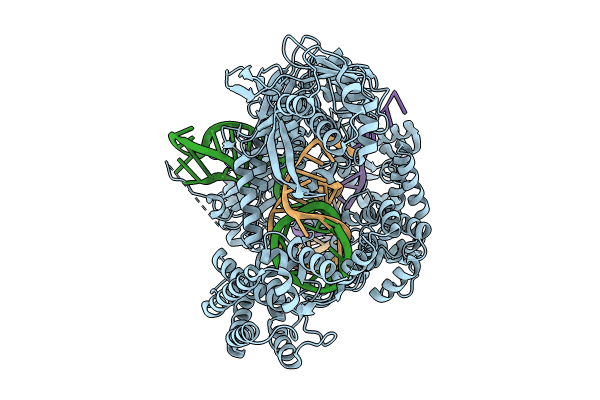
PDB ID:
8SFP
Keywords:
Title:
WT CRISPR-Cas12a with the target strand in the RuvC active site.
Biological Source:
Source Organism(s):
Acidaminococcus sp. BV3L6 (Taxon ID: 1111120)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.80 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


