Deposition Date
2023-04-09
Release Date
2024-04-17
Last Version Date
2025-02-26
Entry Detail
PDB ID:
8SEF
Keywords:
Title:
Crystal structure of Cy137C02, a monoclonal antibody isolated from macaques immunized with an Epstein-Barr virus glycoprotein 350 (gp350) nanoparticle vaccine
Biological Source:
Source Organism(s):
Macaca fascicularis (Taxon ID: 9541)
Expression System(s):
Method Details:
Experimental Method:
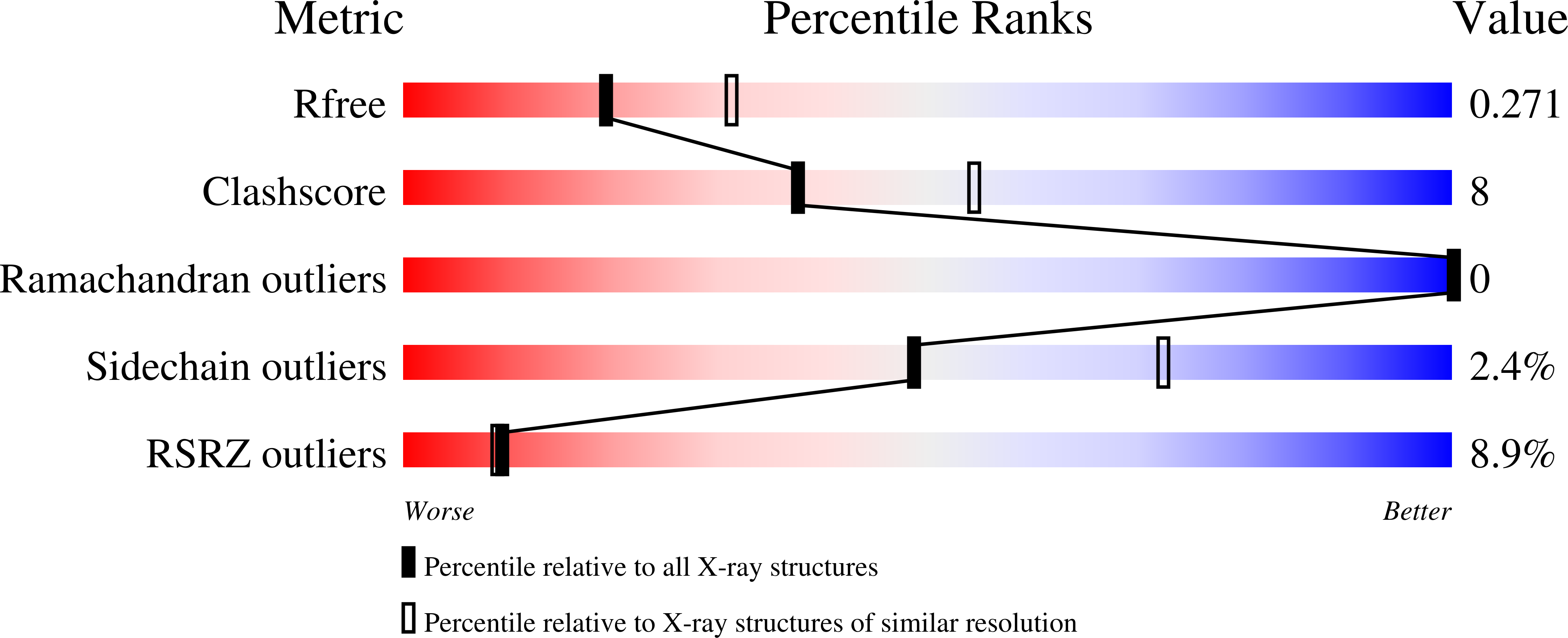
Resolution:
2.47 Å
R-Value Free:
0.26
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
C 1 2 1