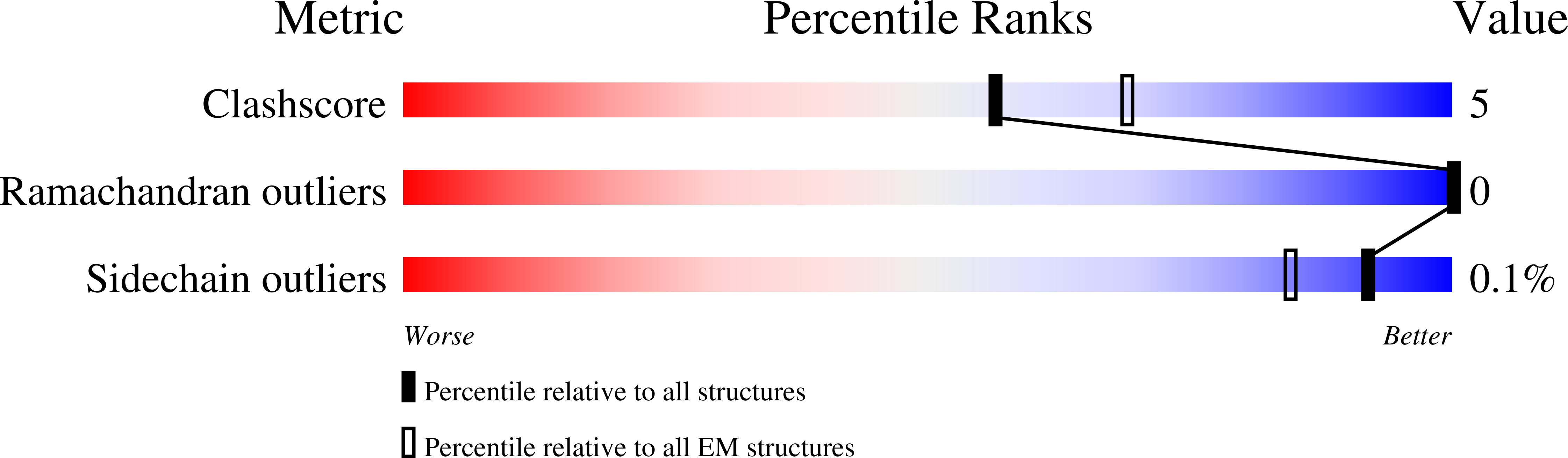Deposition Date
2024-02-01
Release Date
2024-09-04
Last Version Date
2024-10-30
Entry Detail
PDB ID:
8RVN
Keywords:
Title:
Nipah virus (NiV) fusion protein in complex with neutralizing Fab92
Biological Source:
Source Organism(s):
Henipavirus nipahense (Taxon ID: 3052225)
Oryctolagus cuniculus (Taxon ID: 9986)
Oryctolagus cuniculus (Taxon ID: 9986)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.50 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE