Deposition Date
2024-01-11
Release Date
2024-08-07
Last Version Date
2024-11-20
Entry Detail
PDB ID:
8RO0
Keywords:
Title:
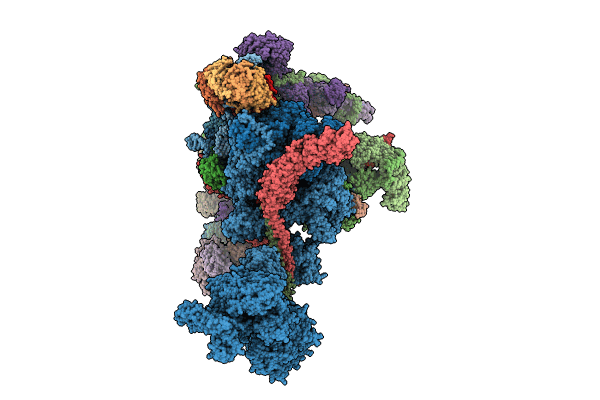
Structure of the C. elegans Intron Lariat Spliceosome primed for disassembly (ILS')
Biological Source:
Source Organism(s):
Caenorhabditis elegans (Taxon ID: 6239)
Method Details:
Experimental Method:
Resolution:
2.90 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE