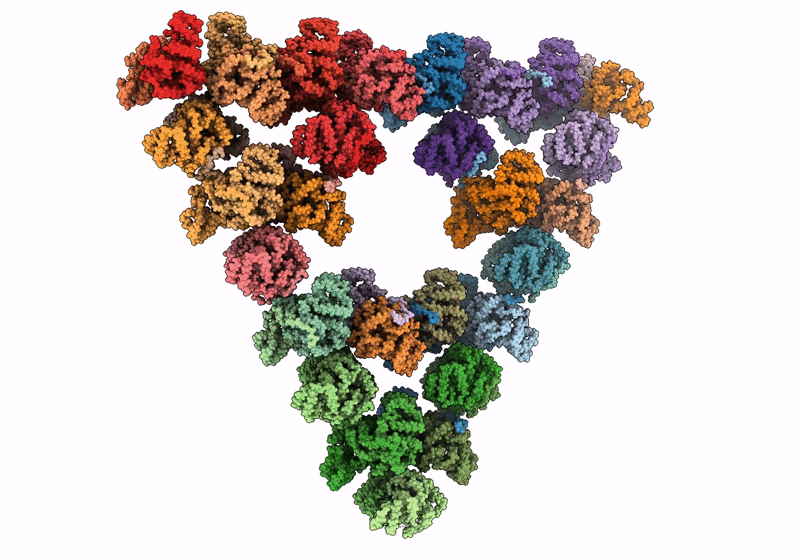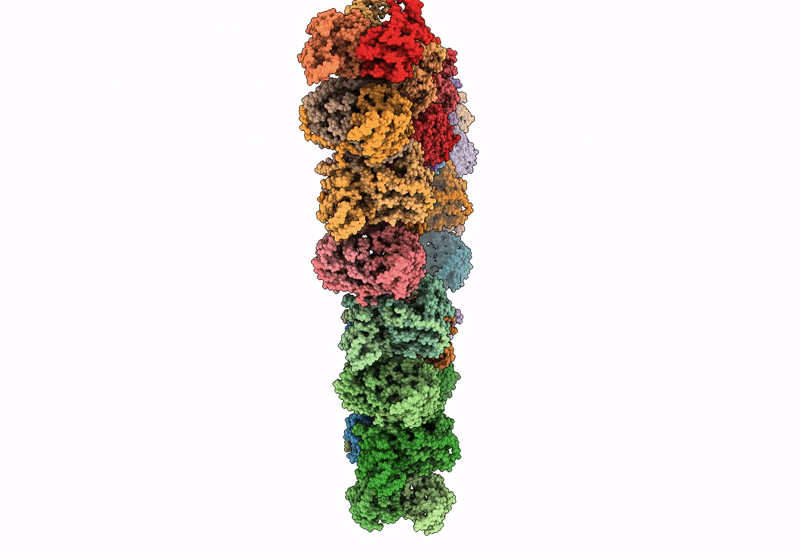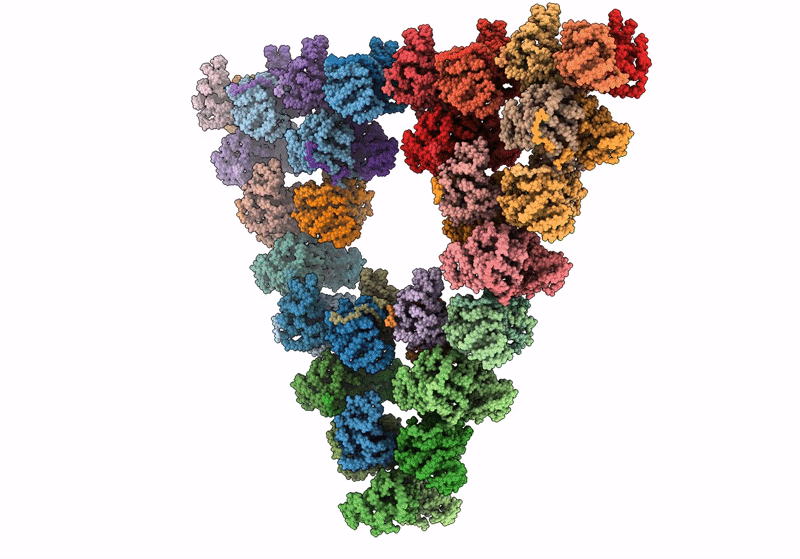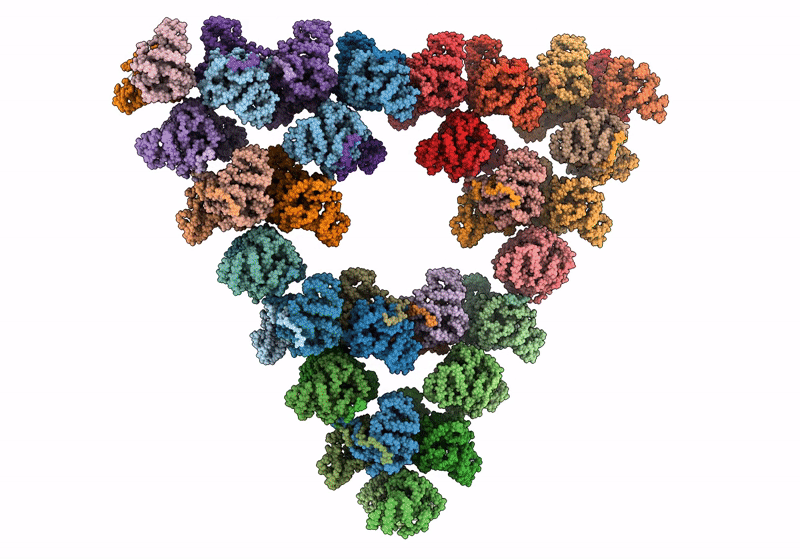
Deposition Date
2023-12-21
Release Date
2024-02-28
Last Version Date
2024-05-08
Entry Detail
PDB ID:
8RJK
Keywords:
Title:
Pseudoatomic model of a second-order Sierpinski triangle formed by the citrate synthase from Synechococcus elongatus
Biological Source:
Source Organism(s):
Synechococcus elongatus PCC 7942 = FACHB-805 (Taxon ID: 1140)
Expression System(s):
Method Details:
Experimental Method:
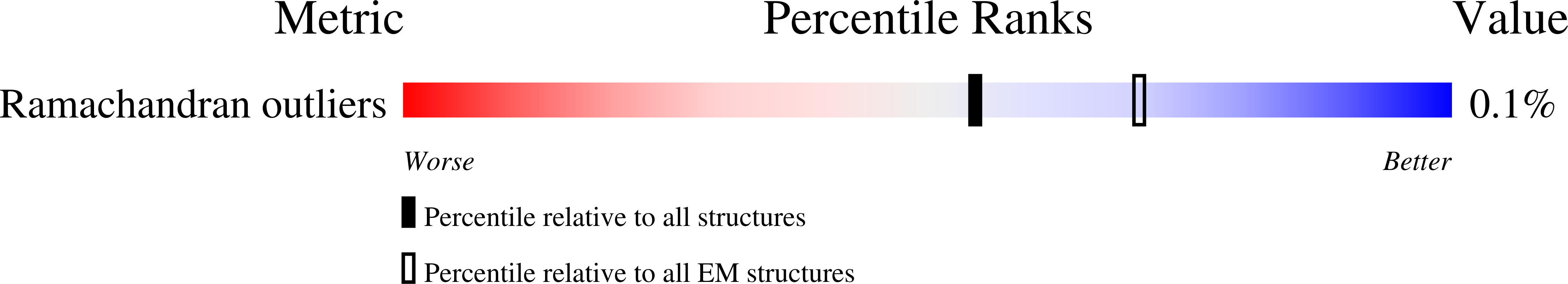
Resolution:
5.91 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE