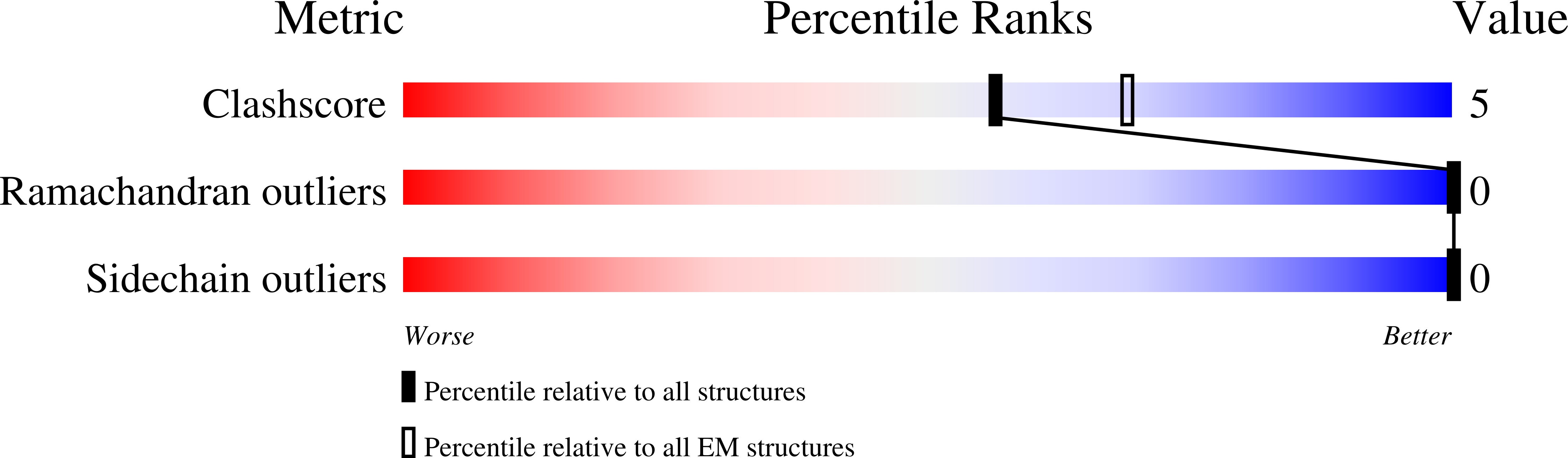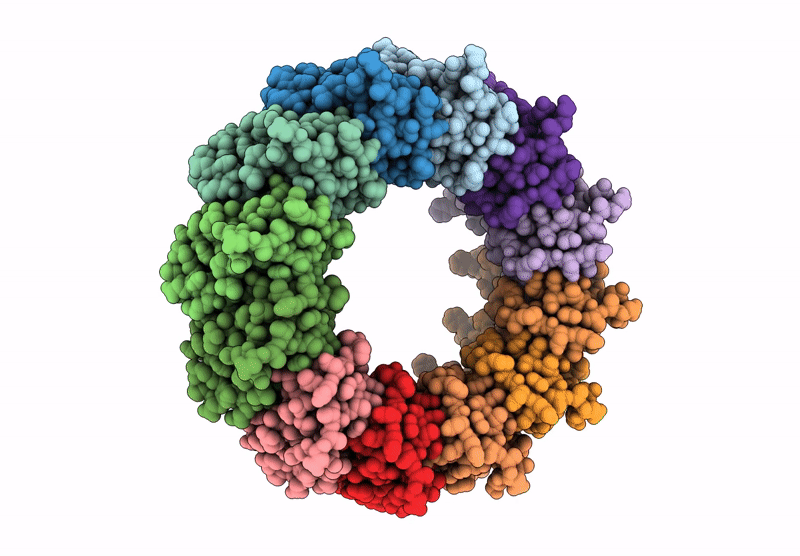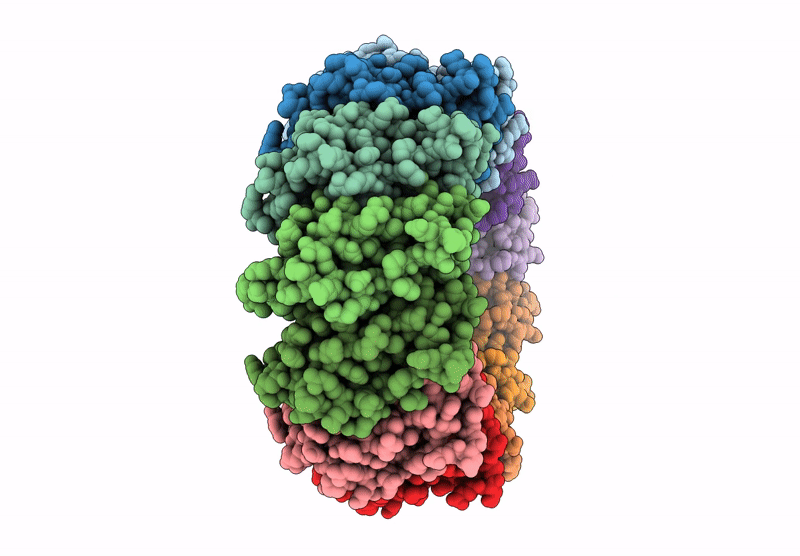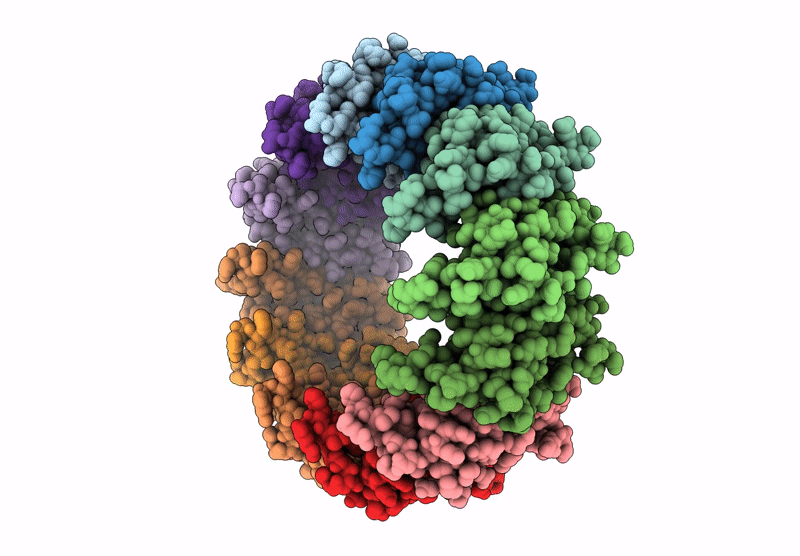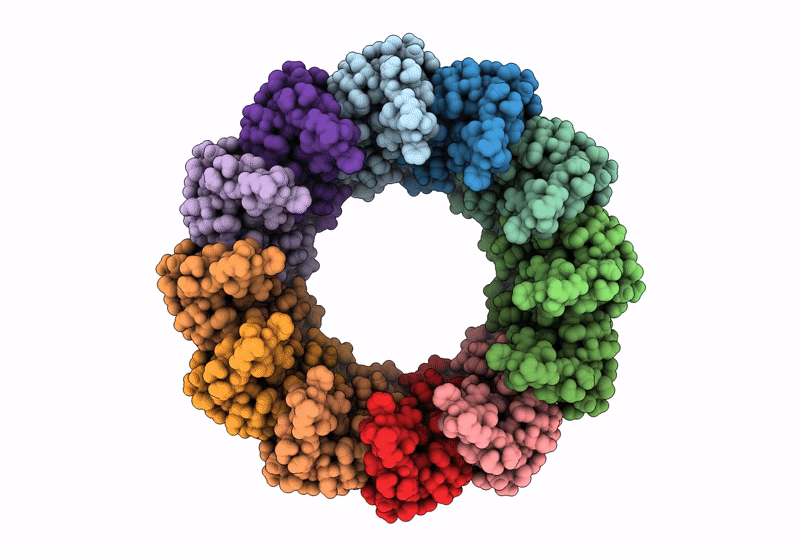
Deposition Date
2023-12-14
Release Date
2025-04-02
Last Version Date
2025-04-09
Entry Detail
PDB ID:
8RGX
Keywords:
Title:
Cryo-EM structure of the Bacterial Proteasome Activator Bpa of Mycobacterium tuberculosis
Biological Source:
Source Organism(s):
Mycobacterium tuberculosis H37Rv (Taxon ID: 83332)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.20 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE